The Effect of Formulation Variables on the Manufacturability of Clopidogrel Tablets via Fluidized Hot-Melt Granulation—From the Lab Scale to the Pilot Scale
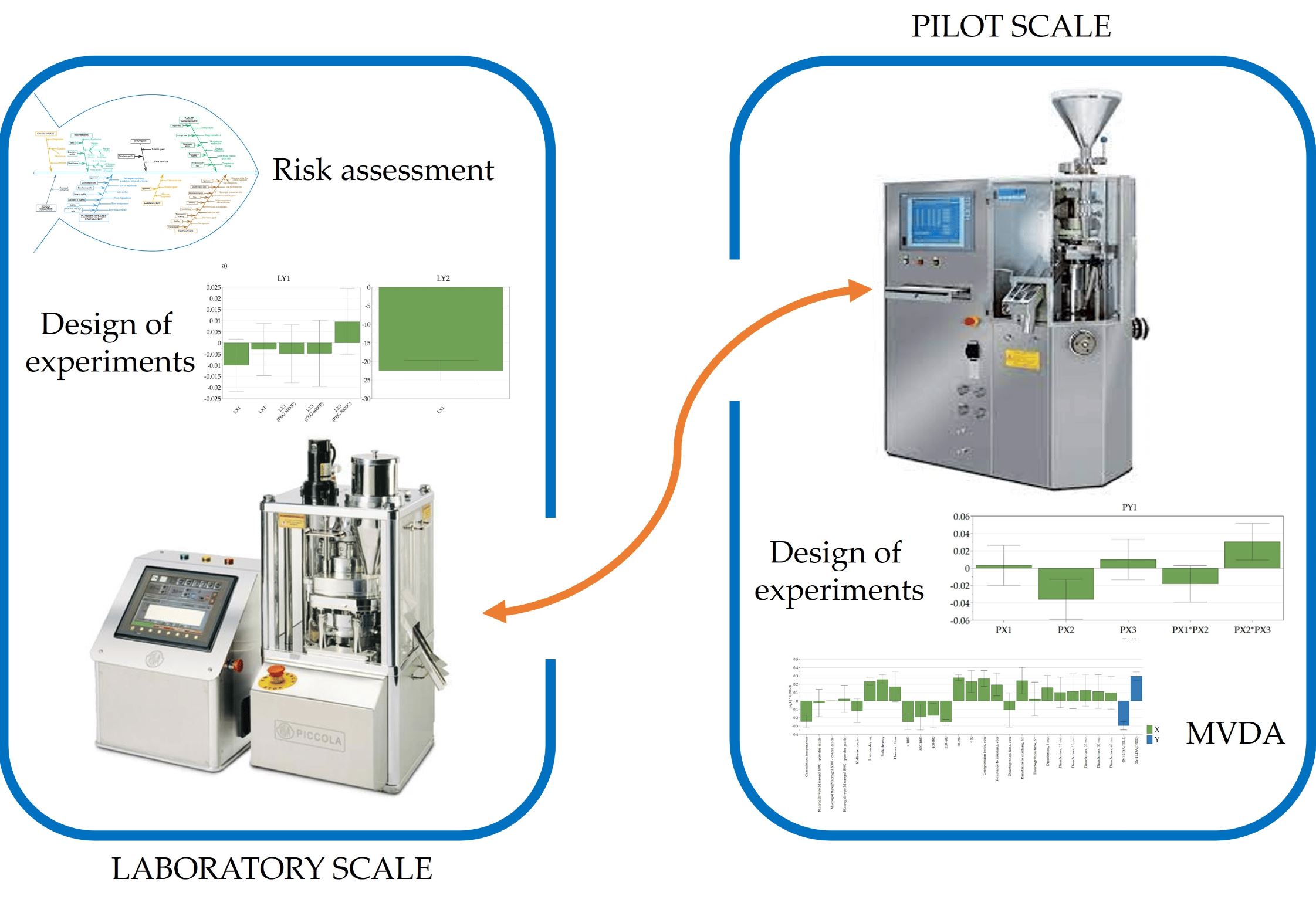
Solid pharmaceutical formulations with class II active pharmaceutical ingredients (APIs) face dissolution challenges due to limited solubility, affecting in vivo behavior. Robust computational tools, via data mining, offer valuable insights into product performance, complementing traditional methods and aiding in scale-up decisions. This study utilizes the design of experiments (DoE) to understand fluidized hot-melt granulation manufacturing technology. Exploratory data analysis (MVDA) highlights similarities and differences in tablet manufacturability and dissolution profiles at both the lab and pilot scales.
The study sought to gain insights into the application of multivariate data analysis by identifying variations among batches produced at different manufacturing scales for this technology. DoE and MVDA findings show that the granulation temperature, time, and Macrogol type significantly impact product performance. These factors, by influencing particle size distribution, become key predictors of product quality attributes such as resistance to crushing, disintegration time, and early-stage API dissolution in the profile. Software-aided data mining, with its multivariate and versatile nature, complements the empirical approach, which is reliant on trial and error during product scale-up.
Download the full article as PDF here: The Effect of Formulation Variables on the Manufacturability of Clopidogrel Tablets via Fluidized Hot-Melt Granulation—From the Lab Scale to the Pilot Scale
or read it here
Materials
The Ph. Eur.-grade active pharmaceutical ingredient Clopidogrel hydrogen sulfate, form II, was obtained from MSN Laboratories Ltd. (Telangana, India). The excipients used for FHMG were Mannitol 35 (Roquette Frères, Lestrem, France) and cellulose, microcrystalline type 103D+ (Mingtai Chemical Co., Ltd., Taoyuan City, Taiwan) as fillers, Macrogol 6000 or Macrogol 8000 (Dow Chemical Company, Hahnville, LA, USA) as plasticizers, and low-substituted hydroxypropyl-cellulose L-HPC, LH-11 (Shin-Etsu Chemical Co., Ltd., Tokyo, Japan), as a binder. Hydrogenated castor oil, Kolliwax HCO (BASF SE, Ludwigshafen, Germany), was used as a lubricant. Analytical-grade potassium chloride, potassium hydroxide, and hydrochloric acid were obtained from Merck (Merck GmbH, Darmstadt, Germany) and used for the preparation of dissolution media.
Equipment
The equipment used for the manufacturing of Clopidogrel tablets at the laboratory scale was as follows:
- Frewitt-Coniwitt Lab rotary sieve with a Ø = 1.0 mm sieve insert (Frewitt Ltd., Fribourg, Switzerland);
- Bosch Solidlab 1 fluid-bed granulator and dryer (Bosch GmbH, Schopfheim, Germany);
- Erweka AR402 double-cone blender (Erweka GmbH, Langen, Germany);
- RIVA Piccola D8 rotary tablet press (RIVA S.A., Ciudadela, Buenos Aires, Argentina);
- Bosch Solidlab 1 film-coating machine (Bosch GmbH, Schopfheim, Germany).
Experiments on the pilot scale were carried out using the following equipment:
- Glatt GS-100 rotary sieve with a Ø = 1.0 mm sieve insert (Glatt GmbH, Binzen, Germany);
- Hüttlin Pilotlab fluid-bed granulator and dryer (Hüttlin GmbH, Schopfheim, Germany);
- Mini Kiskun-Meridián drum blender (Kiskun Meridián Ipari Gépgyártó Zrt., Kiskunfélegyháza, Hungary);
- Korsch XL 100 (Korsch AG, Berlin, Germany);
- GS-HP/F 025 film-coating machine (I.M.A. Industria Macchine Automatiche S.P.A., Ozzano Dell’Emilia (BO), Italy).
Kovács, B.; Tőkés, E.-O.; Kelemen, É.K.; Zöldi, K.; Boda, F.; Suba, E.; Kovács-Deák, B.; Casian, T. The Effect of Formulation Variables on the Manufacturability of Clopidogrel Tablets via Fluidized Hot-Melt Granulation—From the Lab Scale to the Pilot Scale. Pharmaceutics 2024, 16, 391. https://doi.org/10.3390/pharmaceutics16030391
Read also our introduction article on Mannitol here:


