Oral self-nanoemulsifying drug delivery systems for enhancing bioavailability and anticancer potential of fosfestrol: In vitro and in vivo characterization
Abstract
Purpose
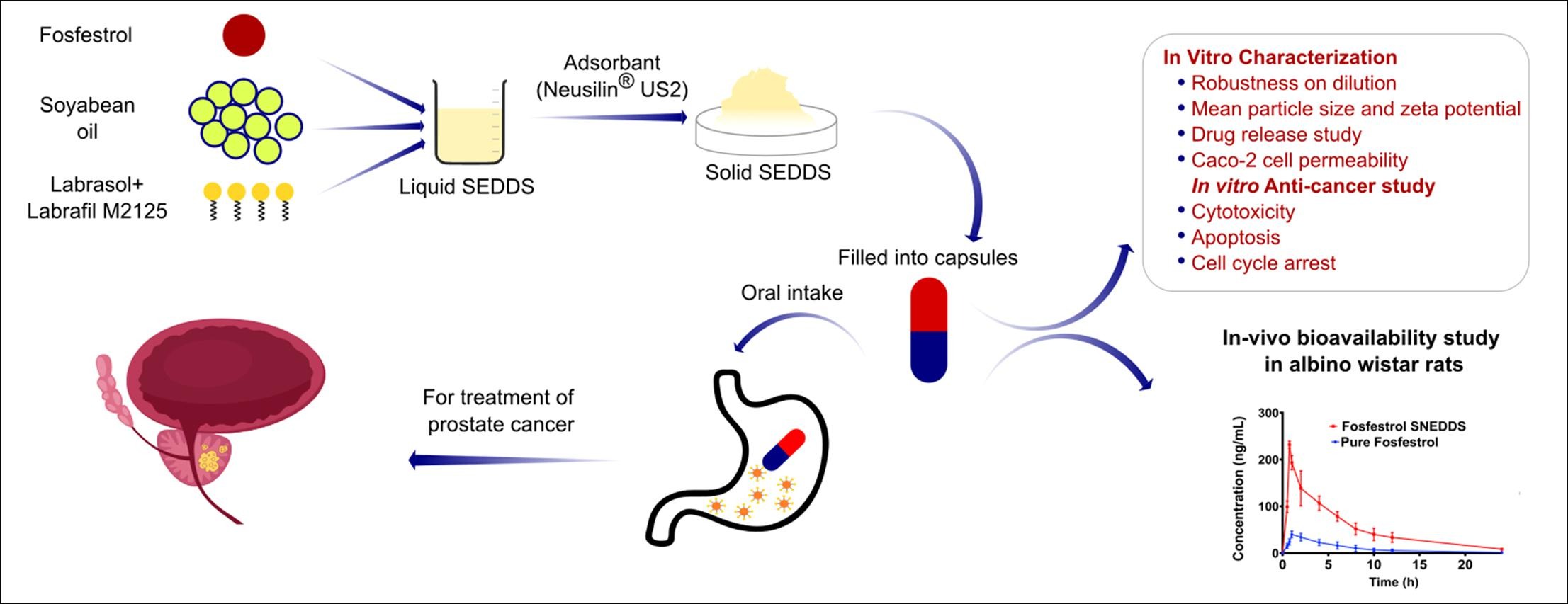
The objective of the current research work was to fabricate a fosfestrol (FST)-loaded self-nanoemulsifying drug delivery system (SNEDDS) to escalate the oral solubility and bioavailability and thereby the effectiveness of FST against prostate cancer.
Methods
32 full factorial design was employed, and the effect of lipid and surfactant mixtures on percentage transmittance, time required for self-emulsification, and drug release were studied. The optimized solid FST-loaded SNEDDS (FSTNE) was characterized for in vitro anticancer activity and Caco-2 cell permeability, and in vivo pharmacokinetic parameters.
Results
Using different ratios of surfactant and co-surfactant (Km) a pseudo ternary phase diagram was constructed. Thirteen liquid nano emulsion formulations (LNE-1 to LNE-13) were formulated at Km = 3:1. LNE-9 exhibited a higher % transmittance (99.25 ± 1.82 %) and a lower self-emulsification time (24 ± 0.32 s). No incompatibility was observed in FT-IR analysis. Within 20 min the solidified FST loaded LNE-9 (FSTNE) formulation showed almost complete drug release (98.20 ± 1.30 %) when compared to marketed formulation (40.36 ± 2.8 %), and pure FST (32 ± 3.3 %) in 0.1 N HCl. In pH 6.8 phosphate buffer, the release profiles are found moderately higher than in 0.1 N HCl. FSTNE significantly (P < 0.001) inhibited the PC-3 prostate cell proliferation and also caused apoptosis (P < 0.001) compared to FST. The in vitro Caco-2 cell permeability study results revealed 4.68-fold higher cell permeability of FSTNE than FST. Remarkably, 4.5-fold rise in bioavailability was observed after oral administration of FSTNE than plain FST.
Conclusions
FSTNE remarkably enhanced the in vitro anticancer activity and Caco-2 cell permeability, and in vivo bioavailability of FST. Thus, FST-SNEDDS could be utilized as a potential carrier for effective oral treatment of prostate cancer.
Materials
Fosfestrol was obtained from Clearsynth Andheri West Mumbai India, Labrasol ®ALF, Labrafil M2125, Cremophor, Labrafac was obtained as a gift sample from Gattefosse India Pvt. Ltd. Mumbai, Soyabean Oil procured from Loba Chemie Pvt. Ltd. Mumbai, Neusilin-US2 kindly gifted by Fuji Chemicals Japan, Span 60, Tween 80 and double distilled water was procured from unique biological Kolhapur. All other chemicals used in the current research work were of analytical grade.
Read more
Sunil T. Galatage, Arehalli S. Manjappa, Durgacharan A. Bhagwat, Rahul Trivedi, Ahmad Salawi, Fahad Y. Sabei, Abdullah Alsalhi, Oral self-nanoemulsifying drug delivery systems for enhancing bioavailability and anticancer potential of fosfestrol: In vitro and in vivo characterization, European Journal of Pharmaceutics and Biopharmaceutics, Volume 193, 2023, Pages 28-43, ISSN 0939-6411,
https://doi.org/10.1016/j.ejpb.2023.10.013.

