Incompatibility of antimalarial drugs: challenges in formulating combination products for malaria
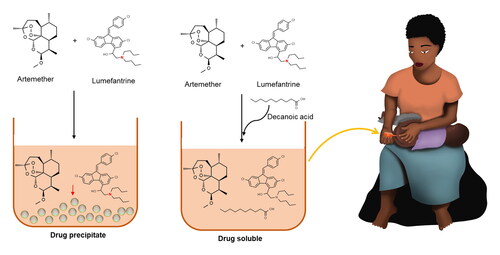
Lipophilic drugs require more advance formulation, especially if the intention is to make solutions or semisolid formulations. This also accounts for most antimalarial drugs. Although some of these antimalarial drugs are soluble in lipid vehicles, few of them, such as lumefantrine (LF), are also poorly soluble in oily vehicles. Trying to dissolve and formulate LF as a liquid formulation together with other antimalarial drugs is, therefore, a major task. When mixed in solution together with artemether (AR), precipitation occurs, sometimes with LF precipitating out on its own, and sometimes with AR precipitating out alongside LF.
In this study, it was hypothesized that the use of fatty acids could lead to enhanced solubility in lipid formulation. Addition of the fatty acid solved the dissolution challenges, making LF soluble for over a year at room temperature (21–23 °C); but further research is needed to test the mechanism of action of the fatty acid. In addition, design of experiments (MODDE® 13) revealed that the amount of fatty acid in the formulation was the only significant factor for LF precipitation.
Read more here
Materials
Trifluoroacetic acid, soybean oil, polyethylene glycol 400, and tetraglycol (Glycofurol) were bought from Sigma Aldrich (Gillingham, UK). Medium chain triglyceride (Labrafac Lipophile WL 1349) was kindly provided from Gattefossé SAS (Saint-Priest Cedex, France). Decanoic acid (DCA) and monounsaturated omega-9 fatty acid (oleic acid) were bought from Tokyo Chemical Industry (Zwijndrecht, Belgium). Miglyol® 812 N was kindly provided from Oleochemical (IOI) Pharma GmbH (Hamburg, Germany). Methoxy polyethylene glycol 350 (Carbowax™ Sentry™) was kindly provided from The Dow Chemical Company (Midland, UK). Extra virgin olive oil (100%) was bought from GEA (Ljutomer, Slovenia). Artemether and LF were kindly provided from Mangalam Drugs and Organics LTD (Mumbai, India). Methanol and acetonitrile were purchased from Honeywell (Seelze, Germany). The water was purified using a Milli-Q water purification system.
Incompatibility of antimalarial drugs: challenges in formulating combination products for malaria, Ellen K. G. Mhango, Benjamin R. Sveinbjornsson, Bergthora S. Snorradottir & Sveinbjorn Gizurarson, Article: 2299594 | Received 27 Mar 2023, Accepted 10 Nov 2023, Published online: 05 Jan 2024, https://doi.org/10.1080/10717544.2023.2299594
Read more on “Orally Disintegrating Tablets (ODTs)” here:


