The R&D landscape for infectious disease vaccines
Vaccines have a tremendous impact on public health, and their importance has been emphasized by the COVID-19 pandemic. Here, we provide an overview of the current state of research and development (R&D) on prophylactic vaccine candidates for infectious diseases globally.
Technology platforms
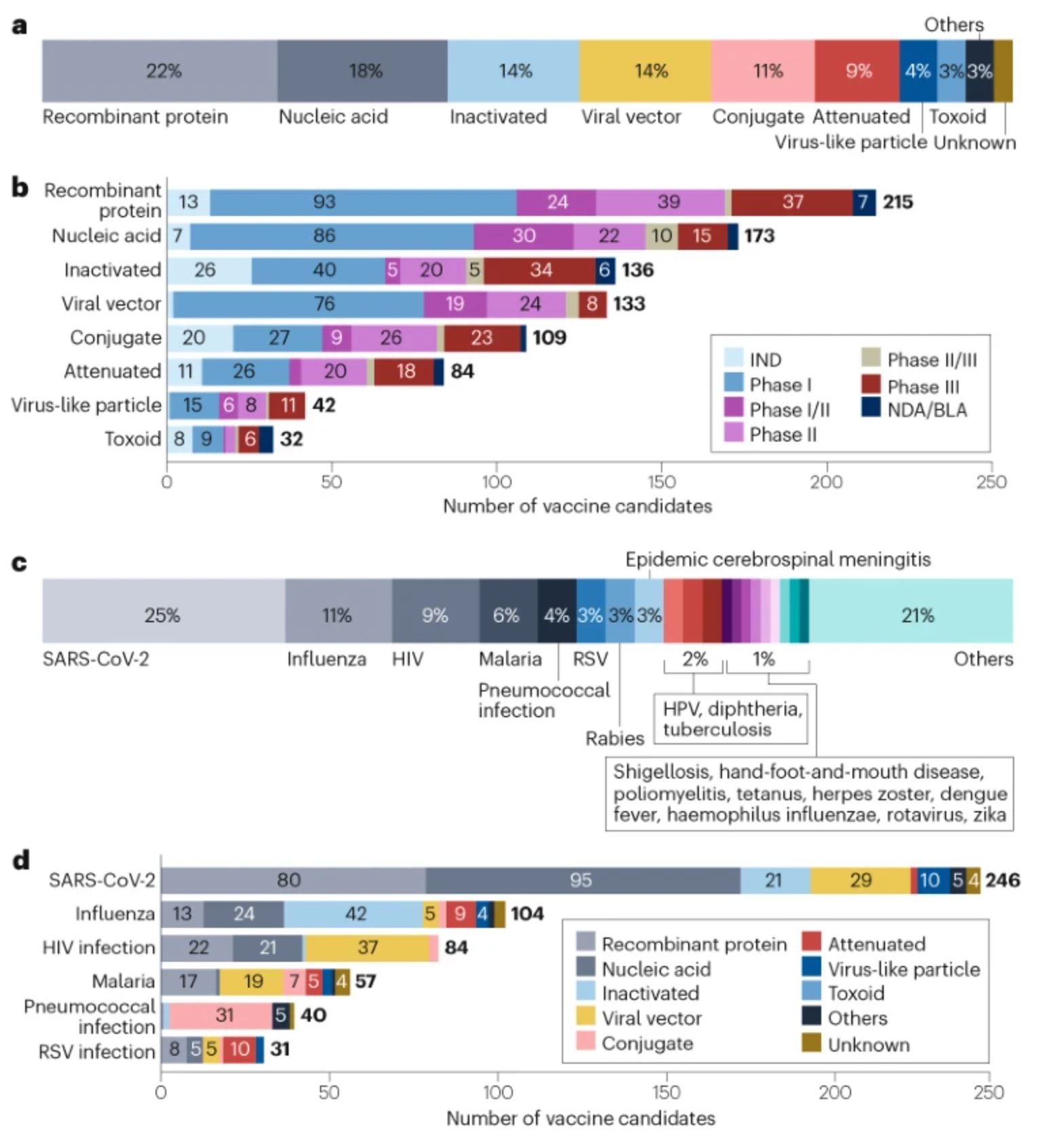
As of 1 January 2023, the global vaccine R&D landscape includes 966 candidates, among which 23% (220) are traditional inactivated or attenuated vaccines (Fig. 1a,b). Advances in molecular technologies have led to the development of other platforms, such as recombinant protein vaccines, nucleic acid vaccines and viral vector vaccines, which have further diversified the pipeline.
Recombinant protein vaccines account for the largest proportion of the pipeline at 22% (215 candidates; Fig. 1a,b), supported by their well-understood safety profile, stability and ease of manufacturing. Almost 100 recombinant vaccine candidates are in phase I development, the highest number at this stage among all the platforms.
The successful launch of SARS-CoV-2 mRNA vaccines has built momentum for nucleic acid vaccine platforms, which include RNA and DNA vaccines. Such platforms now make up the second largest segment of the overall pipeline, at 18% (173 candidates; Fig. 1a,b). Owing to the flexibility of these platforms in developing vaccine candidates for pathogens with high variability in the target antigens, many of these candidates are being developed for such pathogens, including SARS-CoV-2 (95 candidates), influenza (24 candidates) and HIV (21 candidates).
Viral vector vaccines (133 candidates; 14%; Fig. 1a,b) have also garnered attention in recent years, owing to their potential to induce robust and durable immune responses. Various types of viral vectors are being used, including adenoviruses, retroviruses, lentiviruses and poxviruses. In particular, adenoviral vectors (82 candidates) have been widely applied in the development of vaccines for diseases including Ebola, HIV, influenza and SARS-CoV-2. To circumvent the limitation of pre-existing immunity to adenovirus type 5 (Ad5), versatile adenoviral serotypes have been developed, such as Ad26, Ad35 and Ad11.
Conjugate vaccines, which are the next largest group (109 candidates; 11%; Fig. 1a,b), have often been developed against pathogens such as meningococcus, pneumococcus and haemophilus influenzae. These vaccines are based on covalent linkage of immunogenic protein carriers (mostly tetanus toxoid, diphtheria toxoid or group B meningococcal outer membrane protein) to capsular polysaccharides or peptides to enhance immunogenicity and stability.
Continue reading nature reviews drug discovery: https://www.nature.com/articles/d41573-023-00119-4
FROM THE ANALYST’S COUCH, 20 July 2023, The R&D landscape for infectious disease vaccines, Jianying Yue, Yuehua Liu, Mingliang Zhao, Xinzhou Bi, Guanqiao Li & Wannian Liang
Read more articles on “Covid 19” here:
-
The Role of Cyclodextrins in COVID-19 Therapy—A Literature Review
-
An overview on nanoparticle-based strategies to fight viral infections with a focus on COVID-19
-
Inhaled Therapy for COVID-19: Considerations of Drugs, Formulations and Devices