Development of a new inhaled swellable microsphere system for the dual delivery of naringenin-loaded solid lipid nanoparticles and doxofylline for the treatment of asthma
Abstract
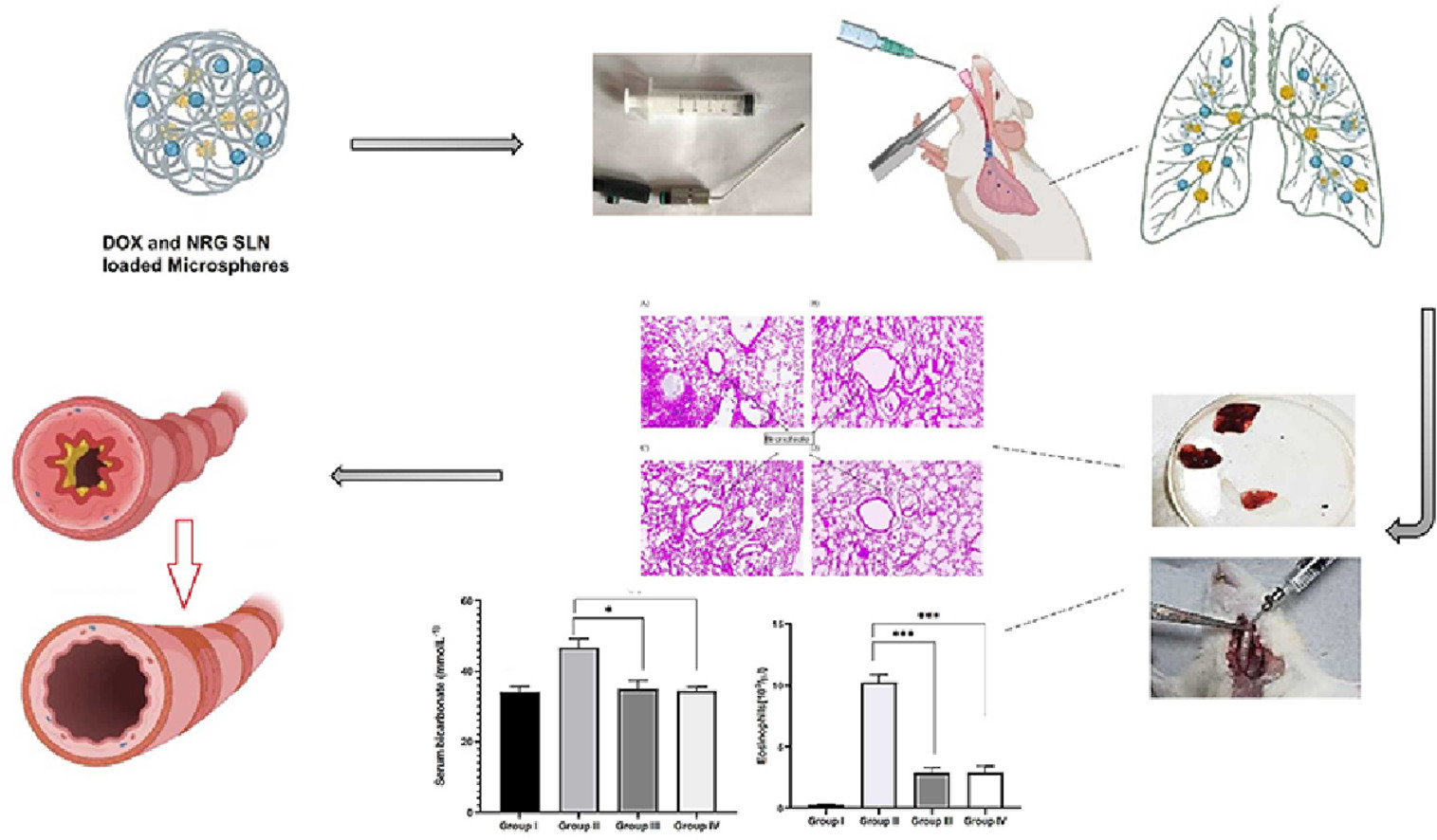
This study developed a new dual delivery system of naringenin (NRG), a polyphenol, and doxofylline (DOX), a xanthine derivative, as an inhaled microsphere system. In this system, NRG has been first loaded into glyceryl tristearate-based solid lipid nanoparticles (NRG SLN), which were further loaded with DOX into swellable chitosan-tripolyphosphate-based microspheres (NRG SLN DOX sMS). The system was characterised based on particle size, PDI, zeta potential, surface morphology (SEM, AFM, and TEM), solid-state and chemical properties (XRD, IR, and NMR), aerodynamic parameters, drug loading, entrapment efficiency and in vitro drug release study. The optimised NRG SLN DOX sMS exhibited particle size, zeta potential, and PDI of 2.1 µm, 31.2 mV, and 0.310, respectively; a drug entrapment efficiency > 79%; a drug loading efficiency > 13%; cumulative drug releases of about 78% for DOX and 72% for NRG after 6 and 12 hours, respectively; good swelling and desirable aerodynamic properties. In addition, in vivo studies conducted in mice, a murine model of asthma showed significant reductions in serum bicarbonate and eosinophil counts and improvement in respiratory flow rate, tidal volume, and bronchial wall lining compared with the asthmatic control group. Overall, this novel inhalable dual-delivery system may represent a good alternative for the effective treatment of asthma.
Introduction
Asthma, a common, non-contagious, chronic inflammatory disorder of the lower respiratory tract, affects people of all ages and can be life-threatening (Bernstein, 2008). About 300 million people worldwide have asthma, and it is estimated to reach 400 million by 2025 (Li et al., 2019). Due to its complicated aetiology, there is no specific treatment for asthma, although long-term systematic treatments can reduce symptoms and attacks and improve prognosis (Ahmad, 2022). Although pulmonary delivery is the most productive approach, it is challenging due to specific barriers, such as airway structure and defense mechanisms (Ibrahim and Garcia-Contreras, 2013). Currently, inhaled corticosteroids are used effectively with short- and long-acting β-agonists and leukotriene receptor antagonists to treat asthma. However, suffering from systemic side effects such as osteoporosis, adrenocortical suppression, Cushing’s syndrome, and the increased risk of infection cannot be ignored (Louie et al., 2013; Liang et al., 2016). Naringenin (NRG) is a polyphenolic flavonoid abundant in citrus fruits and vegetables; it has anti-inflammatory, antifibrogenic, anticancer, and immunomodulatory properties.
NRG has shown therapeutic promise in chronic airway conditions such as asthma and chronic obstructive pulmonary disease (COPD) by modulating the expression of cytokines and chemokines, influencing immune cell differentiation, and mitigating lung smooth muscle remodeling (Xue et al., 2019; Chin et al., 2020; Jasemi et al., 2022). However, water solubility and oral bioavailability are poor and should be improved (Chen et al., 2020). Additionally, Doxofylline (DOX) is a next-generation methylxanthine derivative that acts as a bronchodilator and anti-inflammatory and can replace the use of corticosteroids and theophylline but has better tolerability compared to theophylline (Rogliani et al., 2019; Cazzola and Matera, 2020). The study suggests that DOX exhibits a steroid-sparing effect and is effective at notably low doses when co-administered with steroids in both allergic and non-allergic mice, impacting the lungs (Riffo-Vasquez et al., 2018). However, DOX is taken orally three times a day at a dose of 400 mg. Screening the co-delivery of DOX with NRG has not been reported so far. Therefore, there is a need to improve the delivery system and the route of administration to achieve a better therapeutic efficacy of NRG and DOX over a longer period. To this end, the simultaneous targeted delivery of both drugs to the pulmonary region through nanoscale drug delivery systems may be a promising avenue.
Solid lipid nanoparticles (SLNs) possess the synergistic advantages of various carrier systems such as liposomes, niosomes, etc. They can load lipophilic and hydrophilic drugs and exhibit improved physical and chemical stability. In addition, SLNs display rapid absorption, degradation, and sustained release after pulmonary administration. All these properties make SLN particularly suitable for pulmonary delivery (Li et al., 2021).
Particle size is critical for pulmonary drug delivery. Particles must have an aerodynamic diameter of <5 µm to reach the deep lung through the mouth but should not be finer than 0.5 µm to escape exhalation. In addition, Inhaled particles must have a well-established clearance system to remove deposited particles from the lungs (Li et al., 2019). The ciliated epithelial cells of the upper respiratory tract wipe away particles in the throat or mouth. Alveolar macrophages can remove even the particles deposited in the alveolar region through phagocytosis once they get deposited there. Hence, to achieve sustained drug delivery, the microspheres must be designed to reach the lung area and prevent their uptake by macrophages (El-Sherbiny et al., 2010; Li et al., 2021).
Therefore, swellable microspheres represent a promising strategy as they remain within the inhalation size range when dry and expand to larger geometric sizes once in contact with the epithelial lining fluid, thus successfully avoiding macrophage absorption and clearance from the deep lung (El-Sherbiny et al., 2010).
This study developed a novel dual drug administration system that simultaneously delivers NRG and DOX to the pulmonary region, the first time reported for this combination. First, the NRG-loaded glyceryl tristearate-based solid lipid nanoparticles (NRG SLN) were prepared by varying processing parameters and surfactants at different concentrations to achieve a slow and prolonged release effect of NRG in the lungs (Ji et al., 2016; Li et al., 2021). Glyceryl tristearate and soy lecithin were used as lipids and Kolliphor 188 (P188), Poloxamer 407 (P407), and Tween 80 (T80) as surfactants. Furthermore, NRG SLNs and DOX were co-loaded into chitosan-tripolyphosphate-based swellable microspheres (NRG SLN DOX sMS) using the ionic gelation method (Lupascu et al., 2015; Dragostin et al., 2020). Chitosan is a naturally-occurring cationic polysaccharide with a high molecular weight linked by β-1,4-glycoside bonds. It is biodegradable and biocompatible and has been identified as safe in lung epithelial cells and in vivo (Kean and Thanou, 2010; Feng and Xia, 2011).
The aim of selecting cationic chitosan and anionic tripolyphosphate (TPP) to manufacture swellable microspheres was to develop loosely cross-linked microspheres that allow rapid release of doxofylline for the treatment of asthma exacerbation as well as the sustained release of naringenin.
The dual drug delivery system, NRG SLN DOX sMS, developed here, was evaluated based on zeta potential, particle size, PDI, entrapment efficiency, drug loading efficiency, swelling index, and surface morphology by SEM, AFM, and TEM Furthermore, solid state and compatibility of the drug were accessed by XRD, IR, and NMR, as well as aerodynamic parameters such as geometric standard deviation (% GSD), fine particle fraction (FPF), and mass mean aerodynamic diameter (MMAD) (determined using an Andersen Cascade Impactor). The in vitro drug release study was conducted for 12 hours using simulated lung fluid (SLF). The optimized formulation was screened against an in vivo Murine asthma model using egg albumin and trypsin in mice and a histopathological study. Various in vitro studies have demonstrated the desired aerodynamic and drug release properties for targeted lung delivery, while in vivo studies in mice have revealed the significant efficacy of the developed dual drug delivery system.
Download the full article as PDF here Development of a new inhaled swellable microsphere system for the dual delivery of naringenin-loaded solid lipid nanoparticles and doxofylline for the treatment of asthma
or read it here
Materials
Doxofylline was a gift from Suven Pharmaceuticals (India); naringenin (purity- 98%) was purchased from Otto Chemika; chitosan (average molecular weight, 90–310 kDa; deacetylation degree of 75–85%), Kolliphor® P188 (P188), poloxamer 407 (P407), mannitol, lactose, tripolyphosphate (TPP), glyceryl tristearate (GTS), and Tween 80 (T80) were from Merck Sigma Aldrich (India); soy lecithin, trypsin, and egg albumin were supplied by HI Media Laboratories (India). Dr. Trust’s compressor kit was obtained from a local pharmacy. Unless otherwise stated, all additional reagents were of analytical grade and used exactly as procured.
Ashutosh Pareek, Rupal Kothari, Aaushi Pareek, Yashumati Ratan, Pushpa Kashania, Vivek Jain, Philippe Jeandet, Parveen Kumar, Azmat A. Khan, Amer M. Alanazi, Madan Mohan Gupta, Development of a new inhaled swellable microsphere system for the dual delivery of naringenin-loaded solid lipid nanoparticles and doxofylline for the treatment of asthma, European Journal of Pharmaceutical Sciences, 2023, 106642, ISSN 0928-0987, https://doi.org/10.1016/j.ejps.2023.106642.
Read and watch more on Chitosan as a pharmaceutical excipient here:

