Injectable systems for long-lasting insulin therapy
Abstract
Insulin therapy is the mainstay to treat diabetes characterizedd by hyperglycemia. However, its short half-life of only 4–6 min limits its effectiveness in treating chronic diabetes. Advances in recombinant DNA technology and protein engineering have led to several insulin analogue products that have up to 42 h of glycemic control. However, these insulin analogues still require once- or twice-daily injections for optimal glycemic control and have poor patient compliance and adherence issues. To achieve insulin release for more than one day, different injectable delivery systems including microspheres, in situ forming depots, nanoparticles and composite systems have been developed. Several of these delivery systems have advanced to clinical trials for once-weekly insulin injection. This review comprehensively summarizes the developments of injectable insulin analogs and delivery systems covering the whole field of injectable long-lasting insulin technologies from prototype design, preclinical studies, clinical trials to marketed products for the treatment of diabetes.
Introduction
Diabetes mellitus, commonly known as diabetes, is an endocrine disorder characterized by persistently high blood glucose levels over a prolonged period that can damage nerves and blood vessels and related organs [1]. It has currently no cure and is one of the major global health problems with significant economic burden. It affects approximately 537 million adults (20–79 years, 6.7 %) worldwide, and its prevalence is expected to increase to 643 million by 2030 and 783 million by 2045 [2]. According to U.S. Centers for Disease Control (CDC) National Diabetes Statistics Report published in 2020, nearly 37.3 million Americans, i.e. approximately 11.3 % of the US population, have diabetes, and among which about 5 % of the patients have type 1 diabetes and 95 % of the patients are estimated to have type 2 diabetes [3]. The global economic burden of diabetes was estimated to be $1.3 trillion in 2015 [4]. In U.S., the total cost of diagnosed diabetes was estimated to be $327 billion in 2017, including $237 billion for direct medical costs and the rest for reduced productivity of the diabetic patients [4].
Type 1 diabetes is characterized by pancreatic β-cell destruction leading to absolute insulin deficiency; whereas type 2 diabetes is characterized by insulin resistance with a progressive deficiency in insulin secretion by pancreatic β-cells [5]. Both type 1 and type 2 diabetes manifest persistent elevation of blood glucose level including blood glucose concentration >125 mg/dL and hemoglobin A1c (HbA1c) level, a form of hemoglobin that is covalently bound to glucose, >6.5 % [6]. HbA1c is considered a well-established surrogate for long-term glycemic control and therefore, reduction of HbA1c level to within 4.0 %-5.6 % normal range is the evidence of long-term glycemic control. However, if untreated, both type 1 and type 2 diabetes can cause serious complications including stroke, myocardial infarction, vision loss, amputation, chronic kidney diseases, and mortality [7], [8], [9].
For patients with type 1 diabetes, multiple daily injections of insulin are the only treatment option. For patients with type 2 diabetes, the treatment starts with management of diabetes through change in life-style including healthy eating, weight loss and regular exercise followed by pharmacological intervention with oral metformin monotherapy. If normoglycemia is still not achieved, metformin is given in combination with the following small molecule oral medications: sulfonylurea, thiazolidinedione, DPP-4 inhibitors and SGLT2 inhibitors [10]. However, these oral medications often fail to achieve desired glucose lowering effect, and insulin therapy is eventually included in the treatment regimen when the HbA1c level of type 2 diabetes patients is consistently more than 6.5 % [11]. Given the prevalence of type 2 diabetes, type 2 diabetic patients account for the use of majority of insulin in the market.
Insulin is an anabolic peptide hormone with a short half-life of 4–6 min and it is secreted from pancreatic beta cells. It regulates the blood glucose level in the body by facilitating the absorption of glucose by different tissues such as liver, fat and muscle. One monomer of regular human insulin consists of an A chain (21 amino acids) and a B chain (30 amino acids) linked by two disulfide bridges and one disulfide bond between two amino acids of the A chain. Two monomers form a dimer due to hydrogen bonding and hydrophobic interactions [12], [13] and B chain residues B8, B9, B12, B16, B21 and B23-28 participate in monomer–monomer interaction to form a dimer (Fig. 1) [14], [15]. Three dimers form a hexamer in the presence of zinc ion wherein the histidine residue at position B10 of each monomer co-ordinates with the zinc ion (Zn++) and A13, A14, A17, B1, B2, B4, B13, B14 and B17-19 residues participate in dimer-dimer interaction (Fig. 1) [14], [15].
Insulin remains as the hexamer in glucose regulated secretory vesicles of pancreatic beta cells. High blood glucose concentration causes influx of a large amount of glucose inside the beta cells through glucose transporter GLUT-2, which is preferentially expressed in the pancreatic beta cell membrane [16]. This influx causes over production of cytosolic ATP which promotes the closure of ATP sensitive potassium channels on the cell membrane [17]. As a result, K+ ions are accumulated inside the cells causing depolarization of the plasma membrane which in turn causes opening of the voltage-gated Ca2+ channels [17], [18]. As a consequence, cytosolic Ca2+ concentration is raised and triggers exocytosis process by which insulin containing secretory vesicles fuse with the plasma membrane and secrete insulin hexamer [19]. The insulin hexamer is dissociated into monomer when secreted from the pancreas [20], [21]. The insulin monomer is absorbed into the systemic circulation and reaches adipose and muscle tissues where it binds with its receptor to exert its glucose lowering effects on muscle and adipose tissues [22]. The amino acid residues A1, A5, A19, A21, B10, B12, B16 and B23-25 participate in receptor binding of insulin [14]. In a healthy person, insulin is continually secreted from the pancreas at a nearly constant rate to maintain a constant blood glucose level in the body [23]. This is called basal insulin secretion. After each meal, blood glucose level rises due to food absorption and causes a surge in insulin secretion from the pancreas to facilitate glucose utilization by the body which is called post-prandial insulin secretion [23].
Currently there are two types of insulin available in the market based on their duration of action: rapid-acting or bolus insulins including insulin lispro, aspart and glulisine, which mimic the post-prandial insulin secretion, and long-acting or basal insulins including insulin glargine, detemir, and degludec, which mimic the basal insulin secretion from the pancreas. Rapid-acting or bolus insulins are used after each meal to control the post-prandial rise in blood glucose level whereas basal or long-acting insulins are used to maintain stable blood glucose level during fasting state or between meals. The rapid-acting insulins require multiple injections in a day and long-acting insulins require once-daily injection for short-term glycemic control i.e. blood glucose concentration < 125 mg/dL and long-term glycemic control i.e. HbA1c < 7 %, respectively.
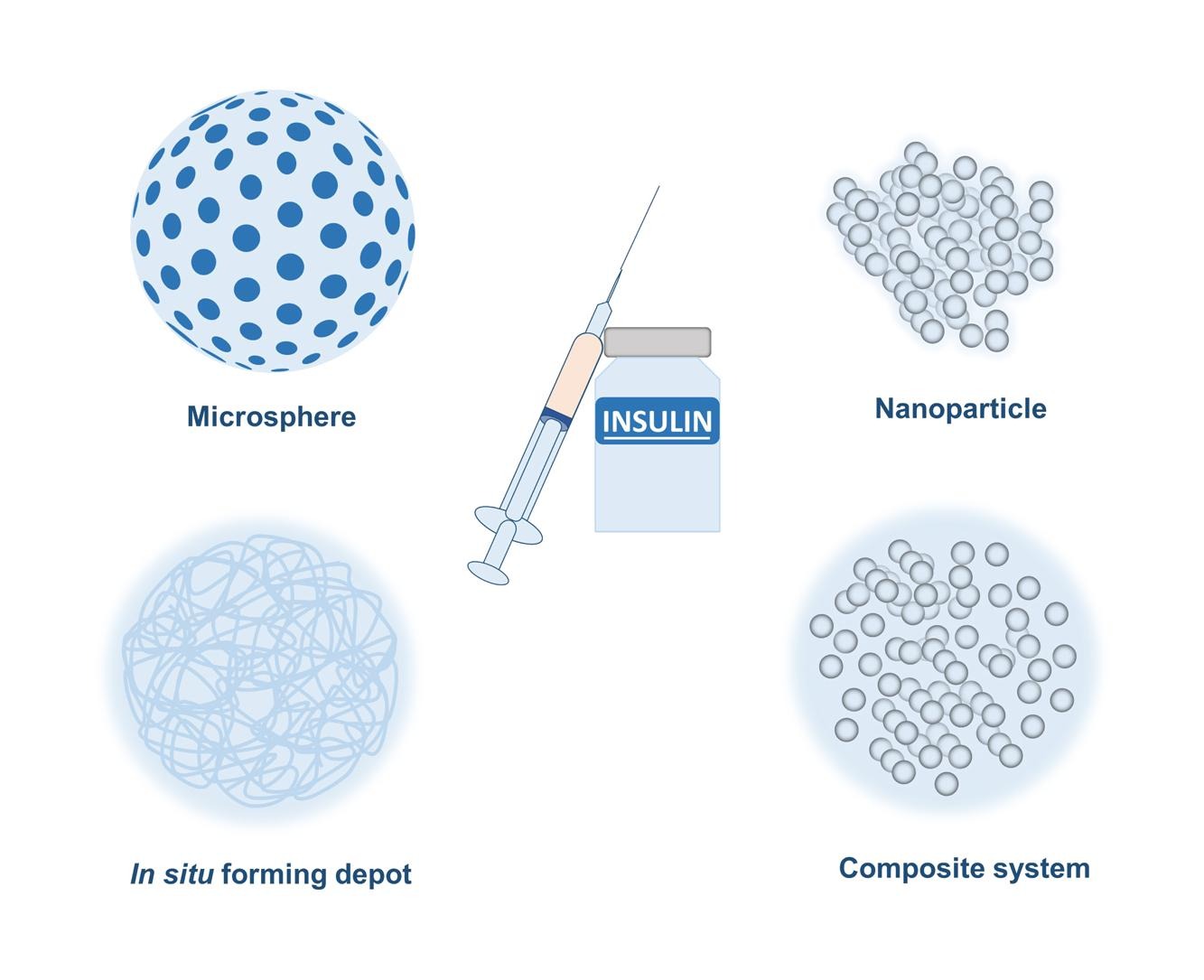
Repeated daily injections can cause serious patient non-compliance leading to non-adherence to treatment and consequently, sub-optimal therapeutic outcomes [24]. To address these issues, substantial efforts have been made in the developments of injectable sustained release systems that can release insulin for days to weeks to maintain normoglycemia and eliminate the need for frequent dosing. Along with more conventional sustained release systems including microspheres and in situ forming depots which have been around for a few decades, several new technologies for sustained drug delivery including nanoparticles, composite systems and glucose responsive systems have also been investigated for sustained insulin release [25]. Currently, a number of products have advanced to clinical trials with the potential to transform the current once-daily basal insulin therapy to once-weekly therapy. This review article comprehensively reviews the current trends and recent advancements in the development of injectable insulin analogs and delivery systems from prototype design and preclinical studies to clinical trials and marketed products as well as provides perspectives on future research and product development in the field of long-lasting injectable insulin therapy.
Read more here
Kumar Kulldeep Niloy, Tao L. Lowe, Injectable systems for long-lasting insulin therapy, Advanced Drug Delivery Reviews, Volume 203, 2023, 115121, ISSN 0169-409X, https://doi.org/10.1016/j.addr.2023.115121.
Read more about diabetes on the World Diabetes Day 2023 :


