Integrated continuous melt granulation-based powder-to-tablet line: process investigation and scale-up on the same equipment
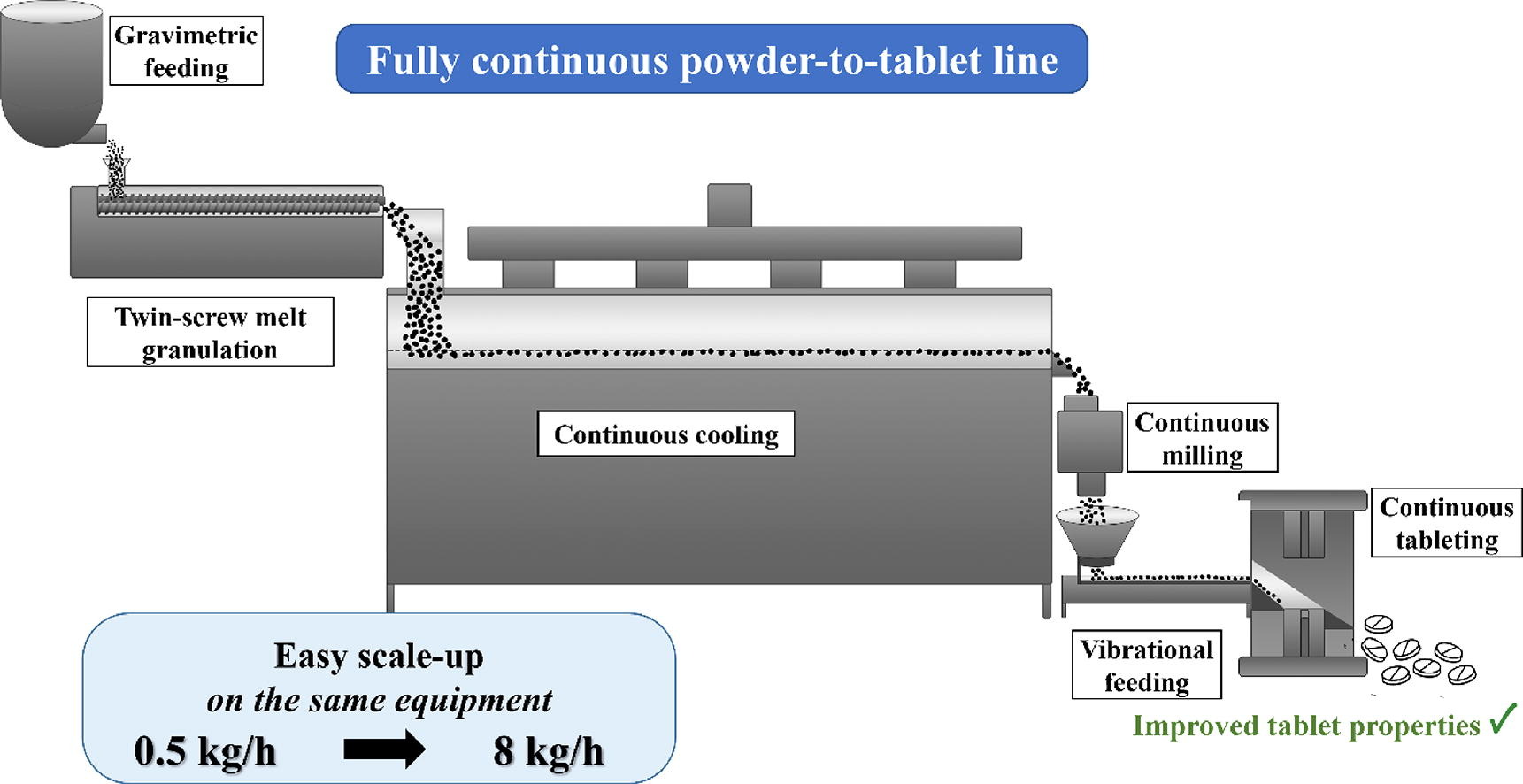
In the last decades, continuous manufacturing (CM) has become a research priority in the pharmaceutical industry. However, significantly fewer scientific researches address the investigation of integrated, continuous systems, a field that needs further exploration to facilitate the implementation of CM lines. This research outlines the development and optimization of an integrated, polyethylene glycol aided melt granulation-based powder-to-tablet line that operates fully continuously. The flowability and tabletability of a caffeine-containing powder mixture were improved through twin-screw melt granulation resulting in the production of tablets with improved breaking force (from 15 N to over 80 N), excellent friability, and immediate release dissolution.
Highlights
- Fully continuous melt granulation-based powder-to-tablet line was developed.
- The flowability and tabletability were improved significantly.
- Caffeine-loaded tablets with increased breaking force (from 15 N to over 80 N) were produced.
- The tablets had excellent friability and immediate release dissolution.
- Scale-up production (from 0.5 kg/h to 8 kg/h) was accomplished with the same system.
The system was also conveniently scaleable: the production speed could be increased from 0.5 kg/h to 8 kg/h with only minimal changes in the process parameters and using the same equipment. Thereby the frequent challenges of scale-up can be avoided, such as the need for new equipment and separate optimization.
Download the full article as PDF here Integrated continuous melt granulation-based powder-to-tablet line_process investigation and scale-up on the same equipment
or read it here
Materials
Our model system comprised caffeine (API) and polyethylene-glycol (binder) as key components. The caffeine-anhydrous and crosslinked polyvinylpyrrolidone (crospovidone, Kollidon® CL) were obtained from BASF (Ludwigshafen, Germany), the lactose monohydrate (Granu-Lac® 230) was provided by Meggle Pharma (Wasserburg, Germany) and polyethylene-glycols with different molecular weight (PEG 3000, PEG 6000 and PEG 20 000) were supplied from Merck Ltd. (Budapest, Hungary). PEG was applied as a melting agent acting as granulation liquid in the granulator at elevated temperatures (>60 °C).
Petra Záhonyi, Fekete Dániel, Edina Szabó, Lajos Madarász, Árnika Fazekas, Anna Haraszti, Zsombor K. Nagy, Integrated continuous melt granulation-based powder-to-tablet line: process investigation and scale-up on the same equipment, European Journal of Pharmaceutics and Biopharmaceutics, 2023, ISSN 0939-6411, https://doi.org/10.1016/j.ejpb.2023.06.005.
Read more on Binder – Pharmaceutical Excipients here:


