The interplay of poorly soluble drugs in dissolution from amorphous solid dispersions
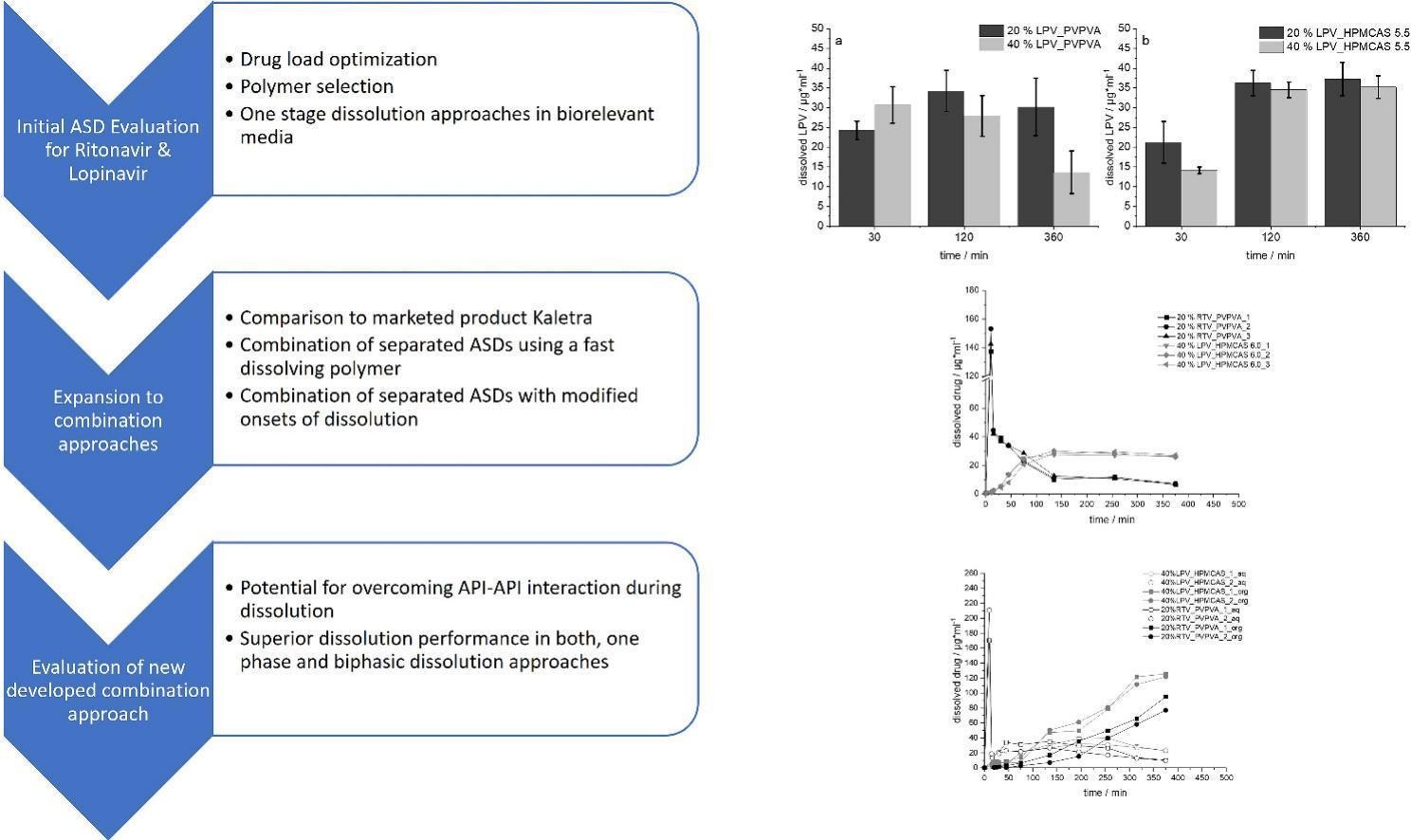
In recent years, the application of fixed dose combinations of antiretroviral drugs in HIV therapy has been established. Despite numerous therapeutic benefits, this approach poses several challenges for the formulation development especially when poorly soluble drugs are considered. Amorphous solid dispersions (ASD) thereby have gained considerable interest in the pharmaceutical field, however, mainly including binary systems containing only one drug and a polymer. The co-formulation of two amorphous drugs can be accompanied by an immense increase in the complexity of the system as exemplarily reported for ritonavir and lopinavir embedded in a composite polymer matrix of PVPVA. The present study aims to present a new formulation approach to overcome the well-documented interaction during dissolution.
Highlights
- Development of a new combination approach for lopinavir and ritonavir.
- Separating the dissolution-onsets was key for the improvement of the overall dissolution performance.
- Embedding lopinavir in different grades of HPMCAS could be used for fine tuning of lopinavir and ritonavir supersaturation.
- Newly developed combination approach also showed superiority in the biphasic dissolution setup.
Two different polymers, PVPVA and HPMCAS were used to produce ASDs for both drugs individually via hot-melt extrusion. The embedding of lopinavir in the slower dissolving polymer HPMCAS, while using PVPVA for ritonavir was found to significantly improve the overall dissolution performance compared to the individual use of PVPVA as well as to the commercial product Kaletra®. In addition, the use of different grades of HPMCAS demonstrated the possibility to further modify the dissolution profile. For a preliminary biorelevant assessment, the selected formulations were tested in a biphasic dissolution setup.
Download the full article as PDF here The interplay of poorly soluble drugs in dissolution from amorphous solid dispersions
or read it here
Materials
The model APIs for this study were RTV (Desano Pharmaceuticals, China) and LPV (Arene Life Sciences, India). The polymers for the preparation of the ASDs were either Kollidon® VA 64 fine (PVPVA, BASF, Germany) or the three different grades of AQOAT® LMP, MMP and HMP (HPMCAS, Shin Etsu, Japan). The film-coated tablet Kaletra® 200/50 mg was chosen as reference product. Fasted State Simulated Gastric Fluid (FaSSGF) and Fasted State Simulated Intestinal Fluid (FaSSIF) used as biorelevant dissolution media were prepared using ready-to-use powder mixtures (Biorelevant.com, United Kingdom). For each experiment, the media were freshly prepared according to the supplier manual. Decanol (Sigma Aldrich Chemie GmbH, Germany) was utilized as organic phase in the biphasic dissolution setup. Ammonium acetate, methanol HPLC grade and hydrochloric acid (all VWR Chemicals, Germany) were used for the preparation of the mobile phase for HPLC analysis. Polylactide (PLA) (Bavaria Filaments, Germany) was used as material for the additional 3D printed paddle for the biphasic dissolution studies.
Marcel Kokott, Jörg Breitkreutz, Raphael Wiedey, The interplay of poorly soluble drugs in dissolution from amorphous solid dispersions, International Journal of Pharmaceutics: X, Volume 7, 2024, 100243, ISSN 2590-1567, https://doi.org/10.1016/j.ijpx.2024.100243.
See our next webinar:
“Rethinking the development of controlled release formulations and manufacturing processes”
Date: 30th of April, Time: 3:00 pm (Amsterdam, Berlin)


