Inclusion of Medium-Chain Triglyceride in Lipid-Based Formulation of Cannabidiol Facilitates Micellar Solubilization In Vitro, but In Vivo Performance Remains Superior with Pure Sesame Oil Vehicle
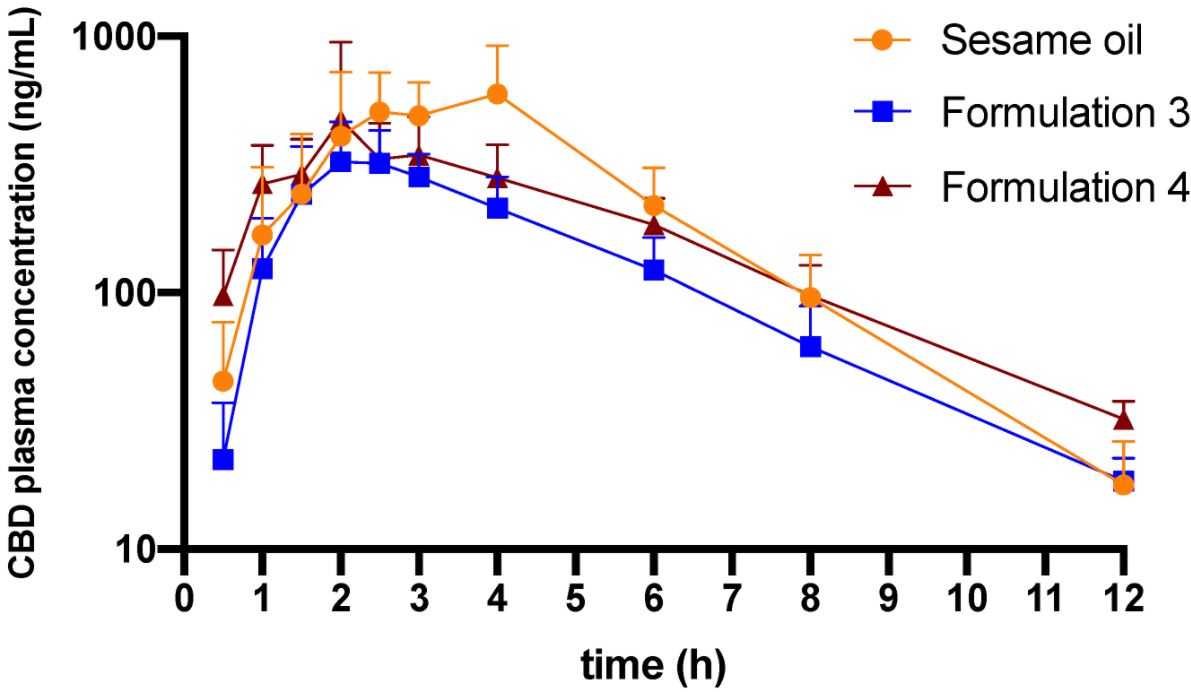
Oral sesame oil-based formulation facilitates the delivery of poorly water-soluble drug cannabidiol (CBD) to the lymphatic system and blood circulation. However, this natural oil-based formulation also leads to considerable variability in absorption of CBD. In this work, the performance of lipid-based formulations with the addition of medium-chain triglyceride (MCT) or surfactants to the sesame oil vehicle has been tested in vitro and in vivo using CBD as a model drug.
The in vitro lipolysis has shown that addition of the MCT leads to a higher distribution of CBD into the micellar phase. Further addition of surfactants to MCT-containing formulations did not improve distribution of the drug into the micellar phase. In vivo, formulations containing MCT led to lower or similar concentrations of CBD in serum, lymph and MLNs, but with reduced variability. MCT improves the emulsification and micellar solubilization of CBD, but surfactants did not facilitate further the rate and extent of lipolysis. Even though addition of MCT reduces the variability, the in vivo performance for the extent of both lymphatic transport and systemic bioavailability remains superior with a pure natural oil vehicle.
or download the full research paper as pdf: Inclusion of Medium-Chain Triglyceride in Lipid-Based Formulation of Cannabidiol
Materials
Feng, W.; Qin, C.; Cipolla, E.; Lee, J.B.; Zgair, A.; Chu, Y.; Ortori, C.A.; Stocks, M.J.; Constantinescu, C.S.; Barrett, D.A.; Fischer, P.M.; Gershkovich, P. Inclusion of Medium-Chain Triglyceride in Lipid-Based Formulation of Cannabidiol Facilitates Micellar Solubilization In Vitro, but In Vivo Performance Remains Superior with Pure Sesame Oil Vehicle. Pharmaceutics 2021, 13, 1349. https://doi.org/10.3390/pharmaceutics13091349
See also our editorial special on excipients for CBD formulation


