The Development of Lipid-Based Sorafenib Granules to Enhance the Oral Absorption of Sorafenib
Abstract
Sorafenib (SFN) is an anticancer multi-kinase inhibitor with great therapeutic potential. However, SFN has low aqueous solubility, which limits its oral absorption. Lipids and surfactants have the potential to improve the solubility of water-insoluble drugs. The aim of this study is thus to develop novel lipid-based SFN granules that can improve the oral absorption of SFN. SFN powder was coated with a stable binary lipid mixture and then absorbed on Aeroperl 300 to form dry SFN granules with 10% drug loading. SFN granules were stable at room temperature for at least three months. Compared to SFN powder, SFN granules significantly increased SFN release in simulated gastric fluid and simulated intestinal fluid with pancreatin. Pharmacokinetics and tissue distribution of SFN granules and SFN powder were measured following oral administration to Sprague Dawley rats. SFN granules significantly increased SFN absorption compared to SFN powder. Overall, the lipid-based SFN granules provide a promising approach to enhancing the oral absorption of SFN.
Introduction
Sorafenib (SFN) is a promising anticancer therapeutic agent approved by the Food and Drug Administration (FDA) for the treatment of patients with advanced hepatocellular carcinoma, advanced renal cell carcinoma, and differentiated thyroid cancer. SFN works as a multi-kinase tyrosine inhibitor, with activity disrupting RAF, vascular endothelial growth factor receptor (VEGFR), and platelet-derived growth factor receptor (PDGFR) kinases [1,2]. The broad pharmacodynamic activity against RAF, VEGFR, and PDGFR kinases provides SFN with extensive antiangiogenic and antitumor proliferative activity against a range of cancers [1,2]. However, SFN is highly lipophilic (LogP 3.8) and insoluble in water (<25 ng/mL). The commercially available sorafenib oral tablet, Nexavar, contains crystalline sorafenib tosylate, a salt form to improve solubility; however, sorafenib tosylate is still insoluble (only 60 μg/mL in water). As a biopharmaceutical classification system class II compound, SFN exhibits poor water solubility, which restricts its absorption into the systemic circulation [3,4]. In addition to poor water solubility, SFN undergoes extensive first-pass metabolism, further reducing its bioavailability [5,6,7]. Clinicians must use frequent high-dosage administration of SFN (400 mg per day) to overcome the low solubility and extensive first-pass metabolism of SFN.
The usage of a high dose of SFN could contribute to toxicity observed in patients such as hand–foot skin reactions, hypertension, and gastrointestinal issues, as well as lesser reported issues [8,9,10,11]. These toxicities can be poorly tolerated by patients, often leading to clinical dose management or complete treatment discontinuation [12,13,14]. With poor oral bioavailability and low tissue exposure levels, SFN is restricted in terms of its therapeutic potential as an oral anti-cancer agent [3,15,16]. Consequently, researchers have been investigating alternative formulation strategies to overcome the poor water solubility of SFN, thereby increasing the oral bioavailability and improving the therapeutic outcome. Improved SFN formulations could produce the same therapeutic effect at lower doses, thereby reducing the associated toxicity risk and presumably increasing patient satisfaction.
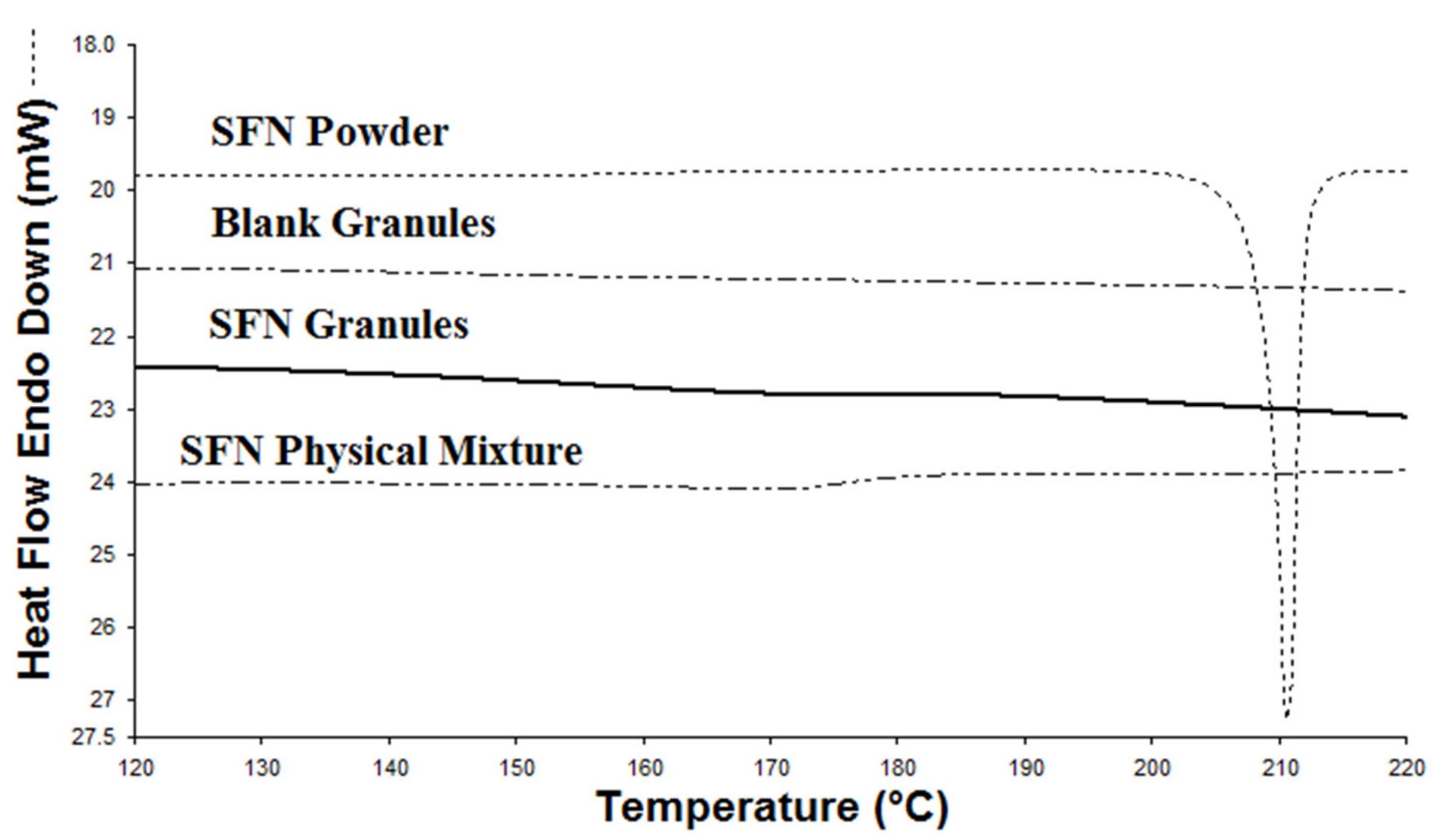
Lipid-based formulations such as solid lipid nanoparticles, microemulsions, and liposomes have the potential to solve the solubility issues of poorly water-soluble drugs. They are prepared from biodegradable and biocompatible lipids and surfactants, reducing likelihood of excipient-safety concerns. However, nanoparticles commonly are made in aqueous phases. Nanoparticles naturally tend to aggregate together over time during storage to decrease their free energy, which can lead to erratic changes in particle behavior and stability [17]. Other lipid-based formulations such as microemulsions and emulsions are also prepared as liquids, leading to issues with stability, the manufacturing process, and costs. Conversion of liquid lipid-based formulations to solid dosage forms is desirable; however, low drug loading (DL) in final solid forms, complex drying procedures, and size increase after drying have hindered such conversion for oral medication formulations. To overcome these issues and use lipid-based formulations in solid dosage forms for oral administration, our lab discovered a new approach to preparing lipid-based solid drug granules with high DL [18,19,20,21]. Recently, we confirmed that the binary lipid mixture of Miglyol 812 and D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) we used in the granules formed stable particles that were not impacted by water dilution in a pseudo-ternary phase diagram [20].
In this study, we aimed to prepare novel lipid-based SFN granules to enhance the oral absorption of SFN. We investigated if coating SFN with the stable binary lipid mixture of Miglyol 812 and TPGS will increase oral absorption. The novel SFN granules were characterized by stability in simulated fluids, two-step biorelevant dissolution, physical state, long-term stability, and in vivo studies, including pharmacokinetics and tissue distribution.
Download the full article as PDF here: The Development of Lipid-Based Sorafenib Granules to Enhance the Oral Absorption of Sorafenib
or read it here
Materials
Research-grade SFN free base was purchased from LC Laboratories (Woburn, MA, USA). TPGS was provided as a gift from BASF (Ludwigshafen, Germany). Miglyol 812 (middle-chain triglycerides) was obtained as gifts from Cremer (Eatontown, NJ, USA). Aeroperl 300 (colloidal silicon dioxide) was provided as a gift from Evonik (Parsippany, NJ, USA). Amicon Ultra-0.5 centrifugal filter unit with a molecular weight cutoff of 100 kDa was purchased from Millipore (Bedford, MA, USA). HPLC-grade methanol was purchased from Fisher Scientific (Fair Lawn, NJ, USA).
Mans, J.C.; Dong, X. The Development of Lipid-Based Sorafenib Granules to Enhance the Oral Absorption of Sorafenib. Pharmaceutics 2023, 15, 2691. https://doi.org/10.3390/pharmaceutics15122691
Watch the video below and read more on Vitamin E TPGS here:

