Mucoadhesive nanostructured lipid carriers as a cannabidiol nasal delivery system for the treatment of neuropathic pain
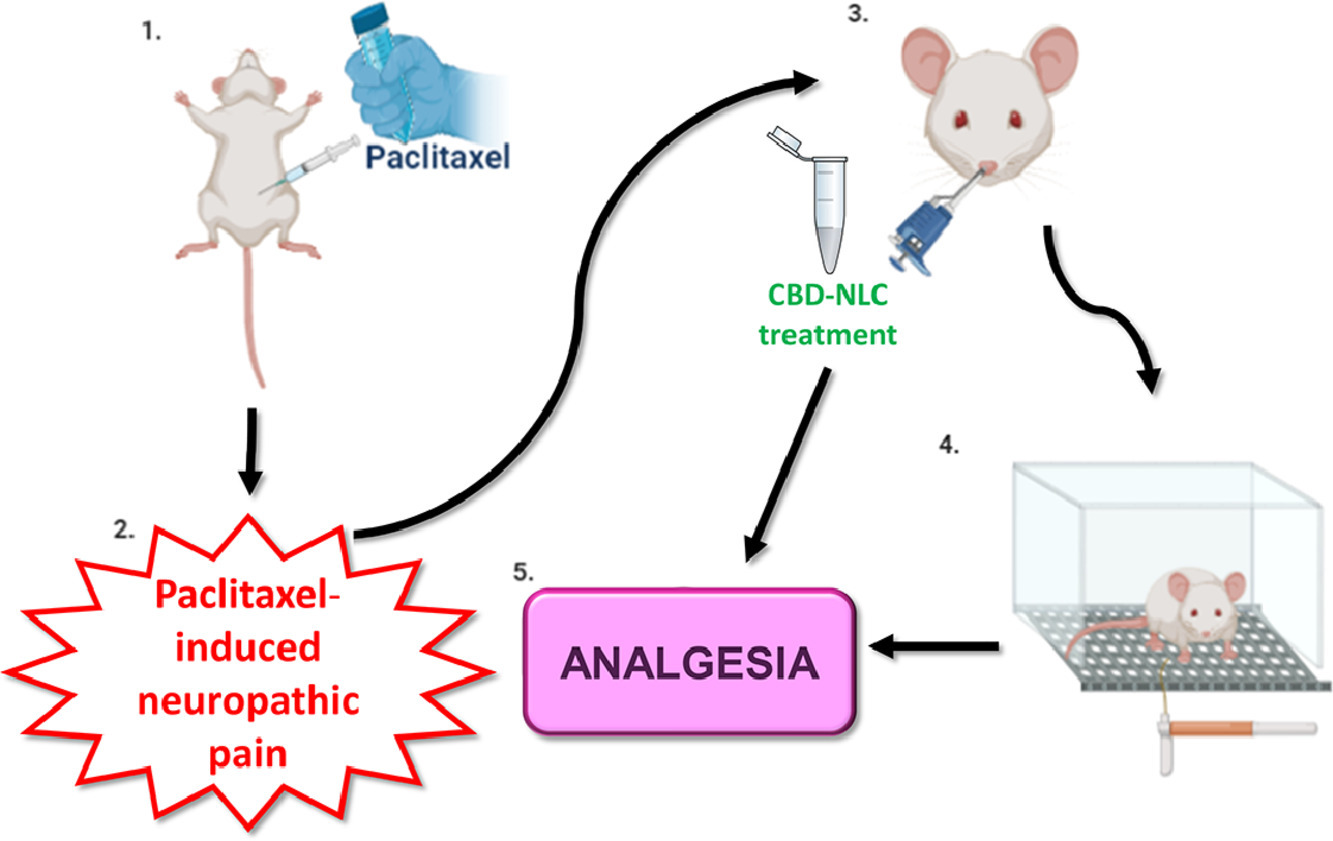
The therapeutic potential of cannabidiol (CBD) has been explored to treat several pathologies, including those in which pain is prevalent. However, the oral bioavailability of CBD is low owing to its high lipophilicity and extensive first-pass metabolism. Considering the ability of the nasal route to prevent liver metabolism and increase brain bioavailability, we developed nanostructured lipid carriers (NLCs) for the nasal administration of CBD.
We prepared particles with a positively charged surface, employing stearic acid, oleic acid, Span 20Ⓡ, and cetylpyridinium chloride to obtain mucoadhesive formulations. Characterisation of the CBD-NLC dispersions showed uniform nano-sized particles with diameters smaller than 200 nm, and high drug encapsulation. The mucoadhesion of cationic particles has been related to interactions with negatively charged mucin. Next, we added in-situ gelling polymers to the CBD-NLC dispersion to obtain a CBD-NLC-gel. A thermo-reversible in-situ forming gel was prepared by the addition of PluronicsⓇ. CBD-NLC-gel was characterised by its gelation temperature, rheological behaviour, and mucoadhesion. Both formulations, CBD-NLC and CBD-NLC-gel, showed high mucoadhesion, as assessed by the flow-through method and similar in vitro drug release profiles.
The in vivo evaluation showed that CBD-NLC dispersion (without gel), administered intranasally, produced a more significant and lasting antinociceptive effect in animals with neuropathic pain than the oral or nasal administration of CBD solution. However, the nasal administration of CBD-NLC-gel did not lessen mechanical allodynia. These findings demonstrate that in-situ gelling hydrogels are not suitable vehicles for highly lipophilic drugs such as CBD, while cationic CBD-NLC dispersions are promising formulations for the nasal administration of CBD. More on Mucoadhesive nanostructured lipid carriers as a cannabidiol nasal delivery system for the treatment of neuropathic pain
What is Span™ 20?
Manufacturer
Croda
Chemical Description
Sorbitan Laurate
Chemical Group: Ester – Sorbitan ester
Product Description
Our Span range of sorbitan esters are widely used as W/O emulsifiers and when used in combination with ethoxylated sorbitan esters (the Tween range) they contribute to the overall stability of O/W emulsions. Manipulation of the Span/Tween ratio produces emulsifying systems of various HLB values, allowing the emulsification of a wide range of oils and waxes. Span 20 finds application in topical preparations. It is soluble in many fatty compositions and solvents and dispersible in water, dilute acids and alkalis. Recommended topical usage levels of 0.5-5%.
Pharmacopoeia Compliance
- FDA-IID Listed
- PhEur
- USP/NF
Route of Administration
- Oral
- Topical
Market Application
- Dermatology
- Human Pharmaceutical
- Veterinary Health

