Lipid solutions for injectables
IOI Oleo GmbH – EXCIPIENTS FOR INJECTABLE FORMULATIONS
For injectable formulations, the selection of a pure excipient is key to ensure constant drug product quality, stability, efficacy, and finally patient safety.
IOI Oleo offers formulators a dedicated range of excipient-grade carrier oils with proven functionality and a trusted reputation with health authorities.
The MIGLYOL® range is regularly and successfully used for the development of IV, SC and IM injections; it meets the applicable quality standards and complies with various current regulations.
CHARACTERISTICS AND TYPICAL PROPERTIES
These oils are highly suitable carriers for poorly soluble APIs for use in injectable formulations. Because of their polarity, they exhibit superior solvent characteristics for some active drugs compared to hydrocarbons.
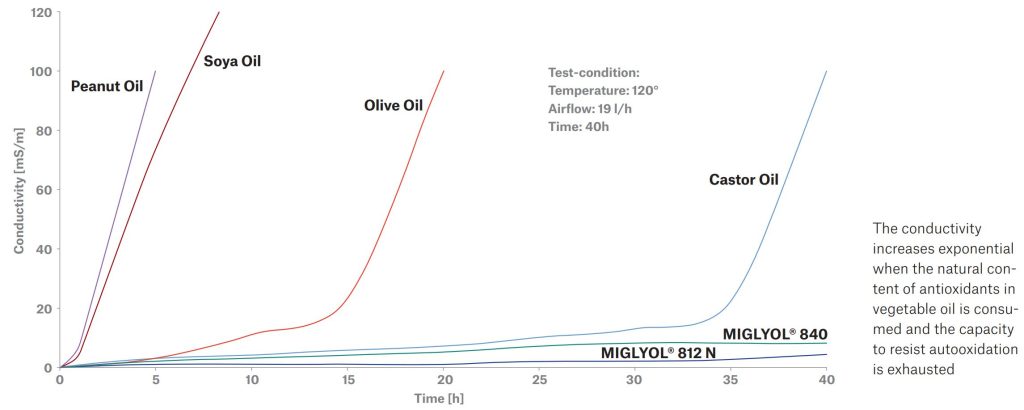
Stability testing on the RANCIMAT repeatedly shows that medium-chain triglycerides exhibit significant advantages with regard to oxidative stability compared to most other typically used pharmaceutical grades of vegetable oils.
MIGLYOL® 810 N and MIGLYOL® 812 N are medium spreading oils and differ in the ratio of C8/C10 fatty acids.
MIGLYOL® 840 is in comparison a higher spreading oil based on propylene glycol as the alcohol component that exhibits extremely low viscosity.
OXIDATION STABILITY WITH METROHM RANCIMAT TEST EQUIPMENT
Additionally, the CEP and Type II DMF can also be referenced if the DRUG SUBSTANCE is used as an exicipient for innovative, high-end injectable dosage forms.
RENEWABLE AND CONSISTENT
Our products are based on:
- High-quality vegetable-derived raw materials
from approved suppliers that comply with tight
specifications and a - GMP-compliant and solvent-free de novo synthesis
This – together with the close monitoring of our manufacturing and purification process – results in products with very high batch-to-batch consistency and advantageous impurity profiles as an ideal basis for drug products with a long shelf life.
PROVEN PHARMACEUTICAL QUALITY
The MIGLYOL® lipid excipients are approved ingredients in injections and infusions:
- Narcotics (Propofol)
- Malaria (Artemether, Lumefantrin)
- Muscle relaxant (Etomidate)
- API-loaded microspheres (Exenatide)
- Veterinary injection suspension
(Amoxicillin, Ceftiofur) - Follicle Stimulating Hormones (Progesterone)
- GnRH Modulator (Triptorelin)
- Neuroleptics (Flupenthixol)
- Benzodiazepines (Diazepam)
See the full brochure on “Lipid solutions for injectables” by IOI Oleo GmbH here:
(click the picture to download the brochure)
Do you need some details or more information? Just fill in the contact form:
Source: IOI Oleo GmbH brochure “Lipid solutions for injectables”