Enabling superior drug loading in lipid-based formulations with lipophilic salts for a brick dust molecule: Exploration of lipophilic counterions and in vitro-in vivo evaluation
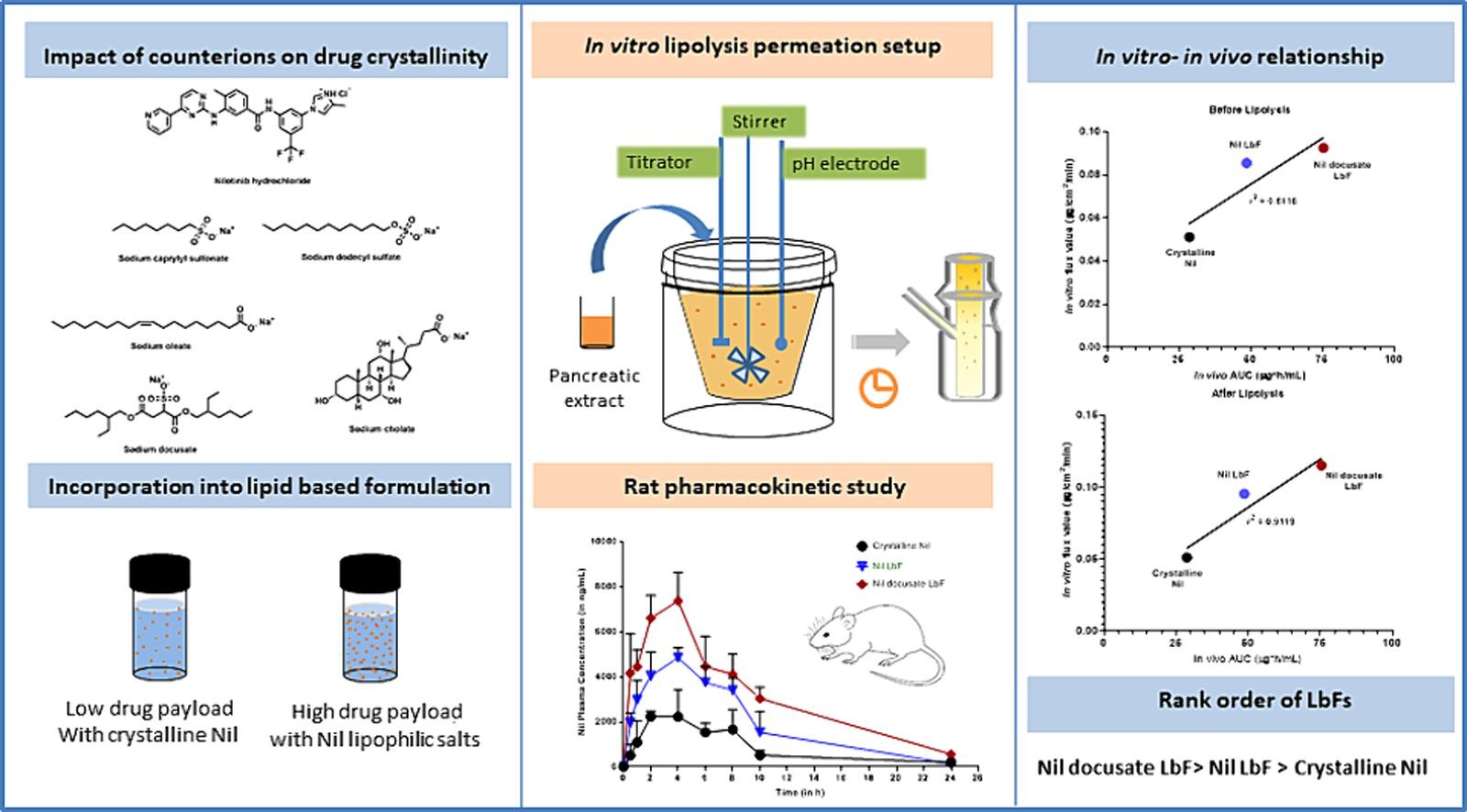
Lipid-based formulations (LbFs) are an extensively used approach for oral delivery of poorly soluble drug compounds in the form of lipid suspension and lipid solution. However, the high target dose and inadequate lipid solubility limit the potential of brick dust molecules to be formulated as LbFs. Thus, the complexation of such molecules with a lipophilic counterion can be a plausible approach to improve the solubility in lipid-based solutions via reducing drug crystallinity and polar surface area. The study aimed to enhance drug loading in lipid solution for Nilotinib (Nil) through complexation or salt formation with different lipophilic counterions.
We synthesized different lipophilic salts/ complexes via metathesis reactions and confirmed their formation by 1H NMR and FTIR. Docusate-based lipophilic salt showed improved solubility in medium-chain triglycerides (∼7 to 7.5-fold) and long-chain triglycerides (∼30 to 35-fold) based lipids compared to unformulated crystalline Nil. The increased lipid solubility could be attributed to the reduction in drug crystallinity which was further confirmed by the PXRD and DSC. Prototype LbFs were prepared to evaluate drug loading and their physicochemical characteristics. The findings suggested that structural features of counterion including chain length and lipophilicity affect the drug loading in LbF.
In addition, physical stability testing of formulations was performed, inferring that aliphatic sulfate-based LbFs were stable with no sign of drug precipitation or salt disproportionation. An in vitro lipolysis-permeation study revealed that the primary driver of absorptive flux is the solubilization of the drug and reduced amount of lipid. Further, the in vivo characterization was conducted to measure the influence of increased drug load on oral bioavailability. Overall, the results revealed enhanced absorption of lipophilic salt-based LbF over unformulated crystalline Nil and conventional LbF (drug load equivalent to equilibrium solubility) which supports the idea that lipophilic salt-based LbF enhances drug loading, and supersaturation-mediated drug solubilization, unlocking the full potential of LbF.
In consideration of these factors, our research aims to synthesize different lipophilic salts/complexes with Nil to disrupt drug crystallinity and compare their solubilization behaviour in aqueous and lipid components. The synthesized lipophilic salts/ complex were incorporated into prototype LbF and evaluated the potential advantage gain in terms of drug loading compared to crystalline Nil. The prototype LbF was a type IIIA-based LbF comprising Capmul® MCM EP (50 % w/v), Kolliphor® RH40 (30 % w/v), and PEG 200 (20 % w/v). Further, the effect of different lipophilic counterions on the dispersion behaviour and aqueous solubilization in the digestive conditions was assessed in vitro. Additionally, we assessed the absorptive flux through the biomimetic membrane and the pharmacokinetic behaviour of prepared formulations. Subsequently, we compared these results with those of unformulated crystalline Nil and conventional LbF. Conventional LbF was prepared by dissolving crystalline Nil in a prototype liquid LbF, where the drug load was equivalent to the equilibrium solubility of Nil in the lipid solution.
Read more here
Arvind Sirvi, Karan Jadhav, Abhay T. Sangamwar, Enabling superior drug loading in lipid-based formulations with lipophilic salts for a brick dust molecule: Exploration of lipophilic counterions and in vitro-in vivo evaluation, International Journal of Pharmaceutics, Volume 656, 2024, 124108, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.124108.
See our next webinar:
“Rethinking the development of controlled release formulations and manufacturing processes”
Date: 30th of April, Time: 3:00 pm (Amsterdam, Berlin)


