How to measure coating thickness of tablets: Method comparison of optical coherence tomography, near-infrared spectroscopy and weight-, height- and diameter gain
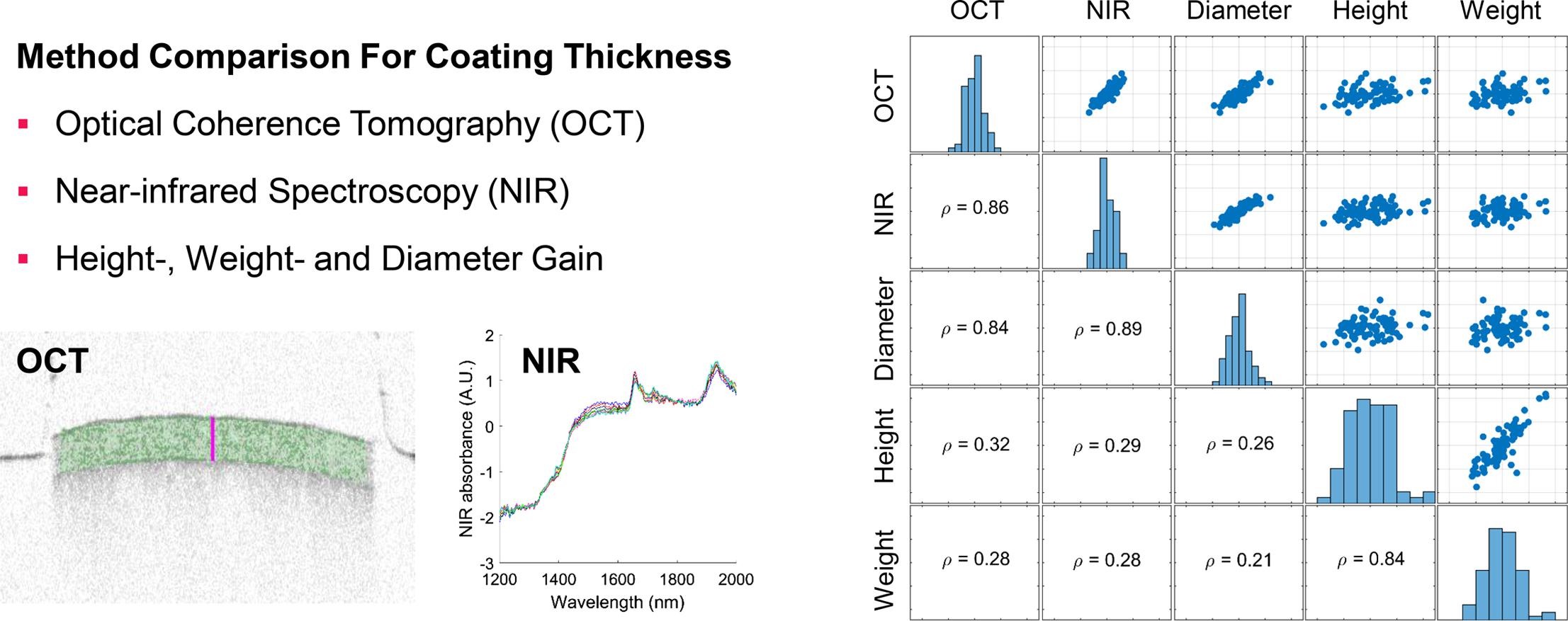
Film coating of pharmaceutical dosage forms, such as tablets and pellets, can be used to tailor the drug release profile. With that regard, a uniform coating thickness of a single tablet (intra-tablet), all tablets (inter-tablet) and subsequent batches (inter-batch) is crucial. We present a method comparison between in-line (optical coherence tomography and near-infrared spectroscopy) and well-established off-line (height-, weight- and diameter-gain) approaches to determining the coating thickness of tablets.
We used single tablets drawn during a commercial coating process. Comparing the low intra– and high inter-tablet coating variability indicated that the tablets had a broad distribution of spray zone passes but at a random tablet orientation. Even at the end of the coating process at a mean coating thickness of about 70 µm, the inter-tablet standard deviation was about 9 µm or 13% relative standard deviation.
Determining correlations between the methods identified the factors that contribute to the measurement uncertainty and bias for each method. Ultimately, we aimed to establish that in-line methods match or even surpass the conventional off-line reference methods in terms of accuracy and precision of coating thickness measurement. Continue reading on Coating thickness measurement of tablets

