Impact testing as a new approach to determine mechanical strength of pharmaceutical tablets
Abstract
One of the most common standardised testing of tablet strength in the pharmaceutical industry is the tablet breaking force, which records data related to diametrical compression. This method does not account for a rapid transfer of energy such as free-falling tablets hitting a solid surface, which occurs throughout manufacture, packaging and shipping. Accordingly, the test shows poor correlation with tablet defect rate. Impact fracture force was identified as a test to measure the force absorbed by the material before fracturing when applying impact energy (dynamic stress). The testing methodology for impact fracture force was modified and developed to characterise pharmaceutical tablets.
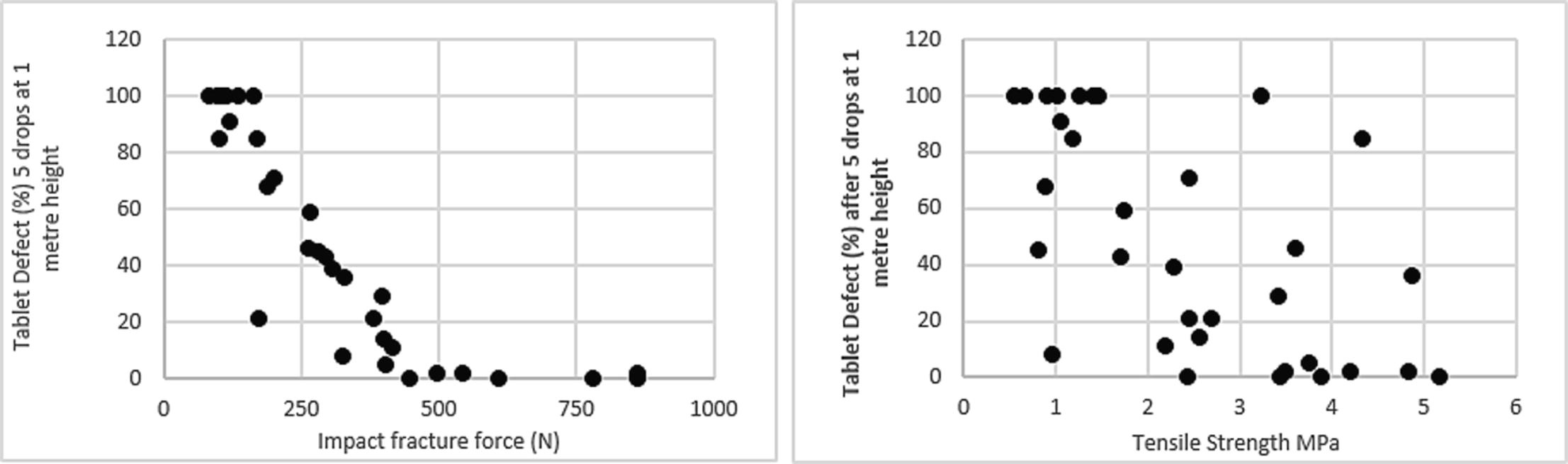
A wide range of tablet formulations with different compositions, sizes, shapes and strengths were evaluated. The results showed that the measured impact fracture force had superior correlation with tablet defect rate in comparison to the standard pharmaceutical tests for breaking and friability with good repeatability. This is the first instrumented impact fracture force tester for pharmaceutical tablets that enables quality by design robust products to withstand and survive mechanical stresses during the manufacturing process. This method has the potential to save extra resource and cost required to solve issues around tablet defects including manufacturing deviations, tablet waste, extra appearance testing, visual inspection and tablet sorting.
Highlights
- Impact testing is presented as a novel approach for determining tablet mechanical strength at scale.
- Impact testing was shown to be superior in its capability to determine the likelihood of defects during large scale manufacturing of tablets.
- Tensile strength cannot be used as the sole indicator of tablet performance.
Introduction
Oral solid dosage (OSD) forms continue to be the preferred route of drug administration for patients, healthcare providers and manufacturers Chein (1992). In 2021 the U.S. Food and Drug Administration (FDA) authorised 53 novel medicines, of which 46 % (23 products) were OSDs Thomas (2022).
Examples of common OSDs include tablets, capsules, powder, beads and lozenges. Tablets are one of the most preferred forms of OSDs as they have a prolonged lifespan, are available in a range of shapes and sizes and can hold a greater dose of an active substance than other OSDs. Further, tablets are simpler and cheaper to manufacture and distribute and easy to administer to patients Awad et al., 2021, Kim and De Jesus, 2023.
Most medicinal tablets are produced by compression process of a powdered or granulated material into a solid dosage Sinka et al. (2009). Subsequent processes may include dedusting, coating and packaging before shipping the final product to markets. In such manufacturing processes, tablets are exposed to various mechanical stresses. These can be caused by the manufacturing process itself, for example tablet ejection from the compression die, shaking the tablet to remove dust or tablet tumbling in a perforated pan coater. Other sources include transferring the tablets to collection containers or loading/unloading manufacturing machines under gravity or vacuum forces. As a result of these forces, tablets collide with each other or against solid surfaces at different speeds, angles and collision numbers. Depending on their mechanical strength, tablets could be prone to complete fragmentations, breakages, or surface chippings. If tablet defect issues are detected during a manufacturing process, they often require extra resource and cost to solve including manufacturing deviations, extra appearance testing, visual inspection and tablet sorting. If these defects are not detected and reach the patients, they could cause more issues related to medicine efficacy, patient safety and compliance and quality reputation damage.
Tablet mechanical strength is a quality attribute criterion for formulation and process optimisation during medicine development and a “in process control” (IPC) in GMP manufacturing (quality control). Standardised testing of tablet strength in the pharmaceutical industry are diametral compression and friability. Both tests are compendial in all major pharmacopeia’s and used often in the regulatory filings and in the manufacturing control strategy. Diametral compression test, sometimes also referred to as tablet breaking force test (USP <1217>) or resistance to fracture test (Ph. Eur. 2.9.8), records data related to diametrical compression force. The tablets are placed between two platens, one of which moves to apply sufficient force to the tablet to cause fracture and the force required to break the tablet is reported. Currently available equipment provides a constant loading rate of 20 N (N) or less per second or a constant platen movement of 3.5 mm or less per second (USP, 2022a). From this test the tensile strength is calculated to provide a more fundamental measure of the mechanical strength of the compacted material that is corrected for the shape and size of the tablet (USP, 2022a).
The scientific relevance of the diametral compression test to the complex and dynamic stresses during tablet manufacturing have been investigated Davies et al., 2007, Hare et al., 2018, Podczeck, 2007, Podczeck, 2012, Sabri et al., 2018. The resistance of tablet to fracture under the influence of manufacturing stresses differs from test conditions for two reasons: firstly, the diametral compression test applies uniaxial stress whereas tablets in manufacturing are exposed to multiaxial stresses Podczeck (2012). Secondly, the rate of which the stress is applied in the diametral compression test is approximately a 1000 times slower than what occurs during manufacturing processes (free falling from a height). The rate of stress application can be a critical factor to tablet failure mode being ductile or brittle and consequently influences their resistance to breakage Davies et al. (1995). Furthermore, the accuracy and link of the diametral compression test to tensile strength of the compacted tablets has been shown to be inaccurate Hilden et al., 2017, Podczeck, 2007, Procopio et al., 2003 and carries up to ∼ 50 % error. Moreover, the diametral compression test has shown poor correlation with tablet defects at simulated manufacturing conditions Gong and Sun, 2015, Hare et al., 2018, Sabri et al., 2018, Wilson and Potter, 1998.
In the friability test, a specified number of tablets are loaded in a rotating drum with certain design and dimensions. The weight loss after 100 rotations is recorded as percentage of the initial tablets weight and reported as the friability. A limit of not more than 1 % without any broken tablets is the pass criterion USP (2022b). The test provides a pass or fail criteria on tablet mechanical strength as the value generated doesn’t link to any fundamental mechanical strength properties of the compact. The generated data is most relevant to the test conditions (fixed drop height and fixed number of drops) and a limited ability to be used to extrapolate beyond the test condition Hare et al., 2018, Podczeck, 2012. Furthermore, the test is considered laborious and material intensive and therefore the application is limited in formulation development space Osei-Yeboah and Sun (2015).
Finding alternative mechanical strength tests for pharmaceutical tablet has attracted researchers over the last few decades Dave et al., 2017, Podczeck, 2012.
In an effort to quantifying brittleness of pharmaceutical tablets Gong and Sun (2015) developed a new tablet brittleness index. The proposed method is based on tablet tensile strength and tablet elastic strain obtained from the standard diametrical breaking test. Commonly used excipient in pharmaceutical tablets with a range of brittle and plastic deformation properties were used to test the proposed method. The index showed to correlate well with the measured tablet friability test.
Hare et al. (2018) proposed a single tablet impact testing to determine tablet propensity for attrition. The impact method was described to directly represent the failure mode tablets may experience during processing under well-defined conditions. In the proposed method, a single tablet was manually dropped from the top of a vertical tube to provide a single impact against a rigid stainless-steel base at a given speed set by the tube height. They also tested a micro indentation methodology to measure tablet mechanical property as a predictor of tablet breakage propensity when exposed to freefall impact. The tested micro indentation method did not provide reliable measurements and no correlation was found with the experimental propensity for breakage.
Osei-Yeboah and Sun (2015) developed an expedited friability method to streamline the test in terms of labour and materials. The developed methodology showed that it can be used to generate data equivalent to those from the standard friability test.
Podczeck (2007) investigated a methodology to compute biaxial tensile strength using data generated from the “ball on ring test”. In this test, the tested tablet is placed on three ball bearing and a biaxial failure stress is loaded through a ball. The biaxial testing was found to be more sensitive than the standard diametral compression test in detecting small changes in the particle characteristics of sorbitol used to make the tablets. Whilst this technique demonstrated increased sensitivity to detect changes in component characteristics, it was not assessed for its capability to determine tablet defects.
Podczeck (2012) reviewed a wide range of tests and methodologies for determination of mechanical strength of tablets including uniaxial compressive strength, direct tensile test, flexural bending and biaxial compression. In this review it was determined that breaking force alone is not an accurate indication of a tablets resistance to defects and that normalisation between different tablet dimensions is required, e.g. conversion to tensile strength. Whilst there are numerous scientific tests that enable the determination of the failure mode of tablets, none have replaced the established tablet breaking force method employed by the USP. This is likely due to either their complex testing method or that the method cannot be applied to all tablet shapes.
Wilson and Potter (1998) investigated three different tablet strength tests (fatigue failure, work of failure, and impact failure) for their potential to provide better correlation with the tablet breakage than the standard diametral compression test. The results generated from the impact test employed showed the strongest correlation with tablet breakage propensity. However, the method utilised was non-instrumented and used a trial and error approach to determine the energy require to cause tablet failure which could have resulted in a degree of variability.
Zhao et al. (2022) proposed a new parameter (Strain/Stress Max) obtained directly from the widely used diametral compression test. They looked to simplify the relationship between the friability test and tensile strength found in previous research Gong and Sun (2015). Several commonly used excipients were used to provide a range of material properties investigated. A strong correlation was found between the (Strain/Stress Max value) and the friability test. Results suggested that tablets that contained a higher ratio of plastic deforming excipients would be beneficial to a given formulation, for it to be able to meet friability criteria. The discovered parameter applicability was only assessed against the standard friability test, which has known limitations in its relation to testing conditions and stresses experienced by tablets during manufacturing.
Ultrasonic measurements Akseli et al., 2009, Akseli et al., 2013, Hakulinen et al., 2008, Leskinen et al., 2010, Simonaho et al., 2011, Stephens et al., 2013 have also been investigated by researchers in an attempt to find an alternative and non-destructive method to determine the mechanical properties of tablets. Ultrasonic techniques are beneficial as they have the potential to provide in line measurements which reduce the need for samples to be removed from the manufacturing process. Whilst these measurements have provided a method to determine dissolution rate or an indication of tensile strength, they have not yet been used to determine a tablet’s likelihood to resist defects during downstream processing.
Albion et al. (2006) quantified the breakage of acetaminophen tablets in a pneumatic transport system using microphones. Tablets were subjected to various velocities during transport with the material of the transport pipes varied. A method was developed in which microphones placed throughout the system were able to detect signals that were suggestive of tablet breakage. Results found that if gas velocity was increased, tablet breakage also increased. A further observation was that steel piping was less likely to induce tablet breakage compared to PVC. The methodology and signal detection poses complexity to application in the wider industry and would need to be calibrated for each specific product.
Researchers have investigated the use of photoacoustic measurements Akseli and Cetinkaya, 2008a, Akseli and Cetinkaya, 2008b, Akseli and Cetinkaya, 2008c, Akseli et al., 2010, Akseli et al., 2008 and X-ray computed tomography Sinka et al. (2004) as a non-destructive technique to determine the mechanical properties of pharmaceutical tablets. Whilst these techniques enable the structure of the tablet to be determined and therefore failure potential, their results have not been compared to established pharmacopeial testing and defect rates on a manufacturing line.
Olaleye et al. (2021) utilised an air gun methodology to determine the breakage of pharmaceutical tablets containing different grades of MCC. In this study, a single tablet was introduced to an air stream of known velocity and impacted against a stainless steel surface. The impact of the tablet was captured using a high speed camera and the pattern of fragmentation was analysed. At set manufacturing conditions, tablets produced that were thicker had higher porosities and were more likely to break. Furthermore, as particle size of the excipient increased, resulting tablets manufactured suffered the same consequence. However, a limited number of excipients were analysed and only one shape was investigated in this study.
Based on the reviewed literature and practice in industry as well as academia, there is a need for a test that can determine a strong correlation and indication of tablet robustness during the manufacturing process whilst also providing digital data with good repeatability, ease of use and accessibility to results.
In this work, a drop-tower impact tester (Instron series 9400) that simulates the type of mechanical stresses that pharmaceutical tablets experience throughout the manufacturing, packaging and shipping processes was investigated. The testing methodology was modified and optimised to characterise pharmaceutical tablets with good accuracy and repeatability. A wide range of tablet formulations with different compositions, sizes, shapes and strengths were evaluated. Multiple tablet drop testing with different height and number of drops were also carried out to characterise the tablets for breakages after freefalling and correlations with the proposed impact testing results were assessed.
Drop tower impact testers are widely used to determine the force and energy required to break or damage a material by the impact of a defined weight striker impacting a specimen with a specified impact energy (determined from the height used and weight of the striker). The technique is widely used to characterise material and component for automobile, aerospace, construction and plastic applications Koffi et al., 2021, Dhakal et al., 2014, Pingulkar et al., 2021.
Read more on the determination of mechanical strength of pharmaceutical tablets
Farhan Alhusban, Euan F. Murgatroyd, Impact testing as a new approach to determine mechanical strength of pharmaceutical tablets, International Journal of Pharmaceutics, 2024, 123891, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.123891.
Read also more on Orally Disintegrating Tablets (ODTs) here:


