Mechanism by Which Magnesium Oxide Suppresses Tablet Hardness Reduction during Storage
Abstract
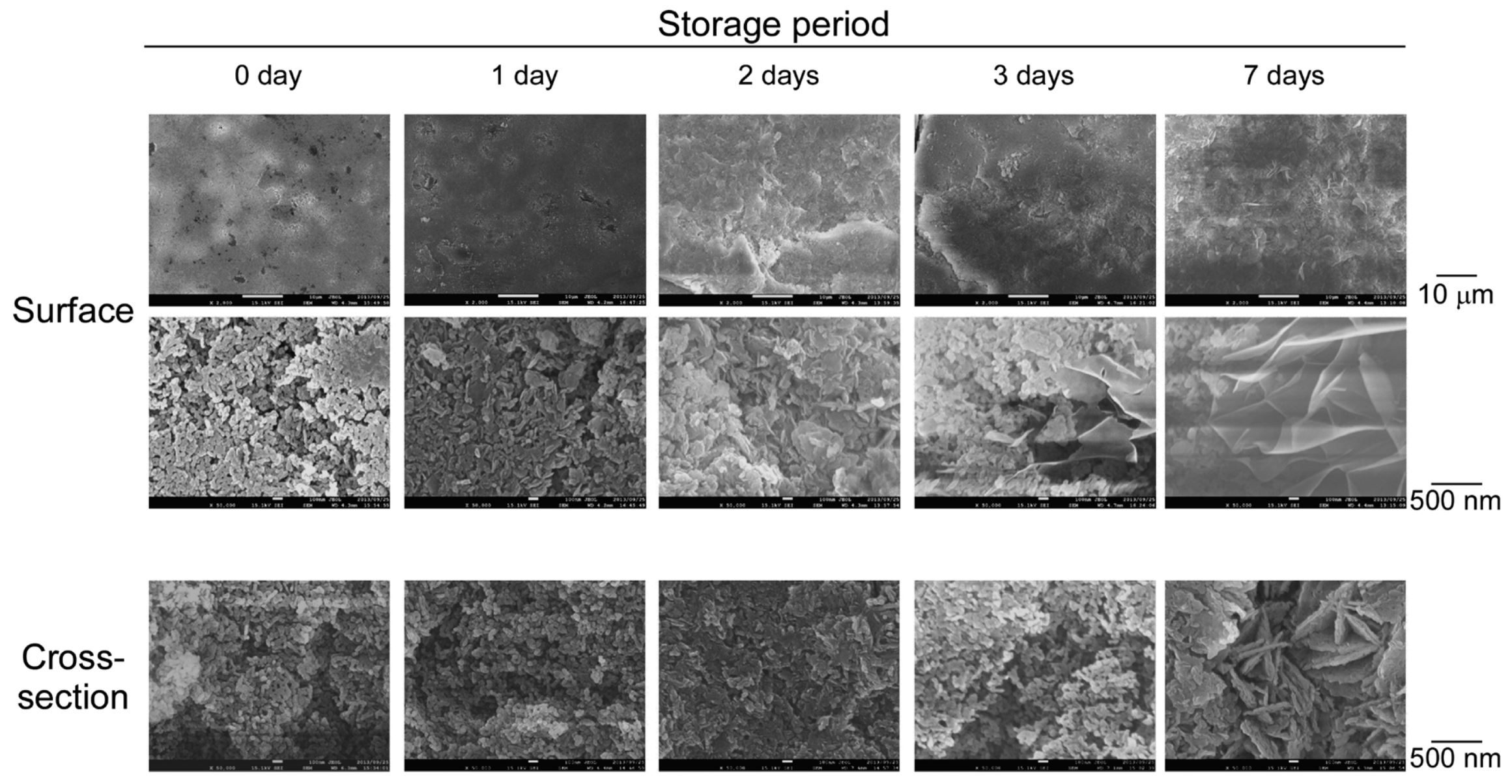
This study investigated how the inclusion of magnesium oxide (MgO) maintained tablet hardness during storage in an unpackaged state. Tablets were prepared with a range of MgO levels and stored at 40°C with 75% relative humidity for up to 14 d. The hardness of tablets prepared without MgO decreased over time. The amount of added MgO was positively associated with tablet hardness and mass from an early stage during storage. Investigation of the water sorption properties of the tablet components showed that carmellose water sorption correlated positively with the relative humidity, while MgO absorbed and retained moisture, even when the relative humidity was reduced. In tablets prepared using only MgO, a petal- or plate-like material was observed during storage.
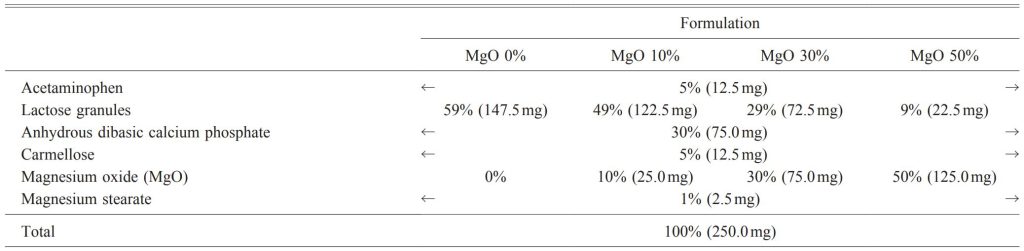
Table 1. Tablet Formulations
Fourier transform infrared spectrophotometry showed that this material was hydromagnesite, produced when MgO reacts with water and CO2. The estimated level of hydromagnesite at each time-point showed a significant negative correlation with tablet porosity. These results suggested that MgO suppressed storage-associated softening by absorbing moisture from the environment. The conversion of MgO to hydromagnesite results in solid bridge formation between the powder particles comprising the tablets, suppressing the storage-related increase in volume and increasing tablet hardness.

a Department of Pharmaceutical Technology, School of Clinical Pharmacy, College of Pharmaceutical Sciences, Matsuyama University; 4–2 Bunkyo-cho, Matsuyama, Ehime 790–8578, Japan: and b Pharmaceuticals Division, Kyowa Chemical Industry Co., Ltd.; 2876–2 Inoue, Miki-cho, Kita-gun, Kagawa 761–0705, Japan. Received January 7, 2016; accepted May 26, 2016, 64_c16-00021.pdf, Adobe Acrobat Document 1.8 MB

