MicroceLac® 100 – MEGGLE’s co-processed lactose grades for direct compression

MEGGLE is a pioneer in co-processing technologies that allow simple, robust formulation development and manufacture. Through co-processing, MEGGLE developed highly functional excipients possessing unique qualities for directly compressible immediate and sustained release pharmaceutical solid dosage forms.
Read the news of MEGGLE´s product MicroceLac® 100 in the following article below and/ or download the technical brochure:
MicroceLac® 100
General information
Direct compression (DC) tablet manufacture is a popular choice because it provides the least complex, most cost effective process to produce tablets compared to other tablet manufacturing approaches. Manufacturers can blend APIs with excipients and compress, making dosage forms simple to produce [1, 2].
DC technology and the use of modern tableting equipment require that excipients and APIs form a compactable mixture with excellent flowability and low particle segregation tendency [3].
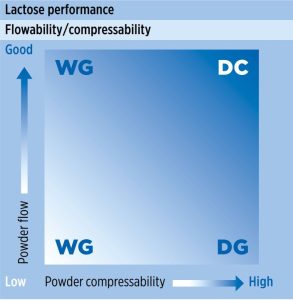
In the pharmaceutical industry, lactose is one of the most commonly used excipients; however, like many other excipients, lactose may not be suitable for direct compression without modification due to insufficient powder flow or/and compaction properties (figure 1).
Product description
Alpha-lactose monohydrate and microcrystalline cellulose are functional excipients used in oral solid dosage forms. Both are naturally derived and commonly used in the pharmaceutical industry, either individually or in combination. To develop synergistic functional performance, such as increased compactability and powder flow, alpha-lactose monohydrate and microcrystalline cellulose were co-spray-dried, creating a monoparticulate system having two compaction mechanisms, brittle fracture and plastic deformation, within individual particles. MicroceLac® 100 provides the flow and compaction properties desired for direct compression tableting. MicroceLac® 100 comprises 75 % alphalactose monohydrate and 25 % microcrystalline cellulose (MCC), both maintaining their individual chemical identities.
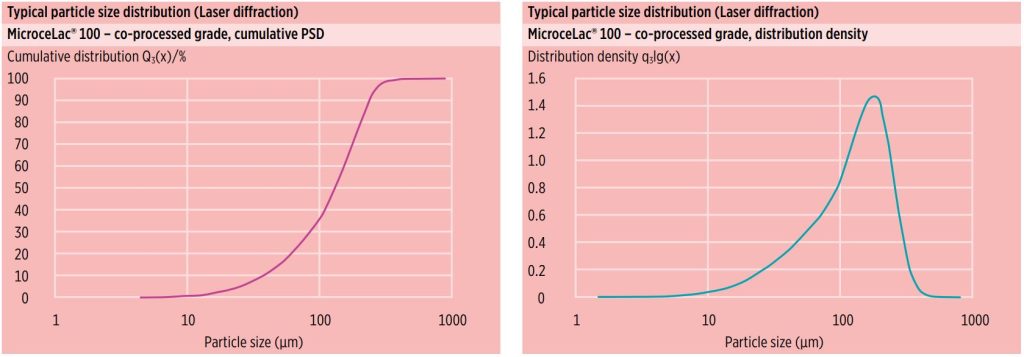
Particle size distribution (PSD)
Figure 2 shows typical laser diffraction particle size distribution data for MicroceLac® 100. MicroceLac® 100 possesses a narrow PSD that supports homogenous powder blend preparation, a requirement for achieving good tablet quality.
Figure 3 depicts the specified PSD range and typical average values by air-jet sieving. These parameters are constantly monitored through in-process control (IPC) testing and are part of the MicroceLac® 100 particle size distribution specification.
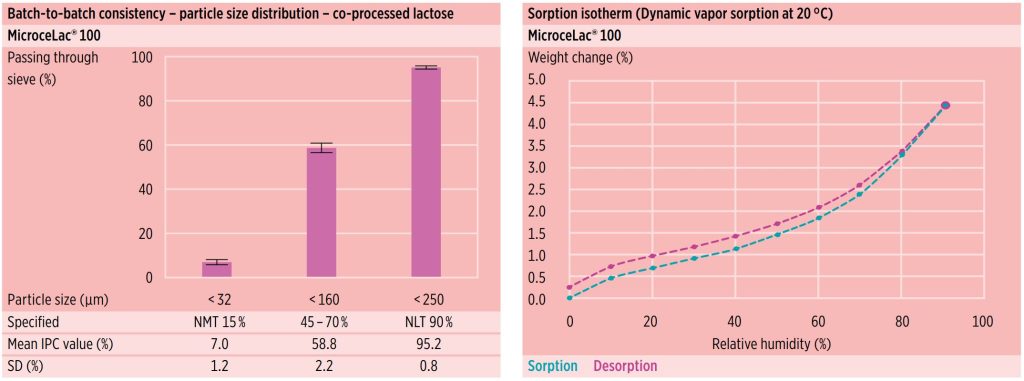
Batch-to-batch consistency
Batch-to-batch consistency for all lactose products can be attributed to MEGGLE’s long history and experience in lactose manufacture, and broad technical expertise. Constant in-process and final product testing ensures consistency and quality (figure 4).
Isotherms
MicroceLac® 100 exhibits moderate moisture uptake while exposed to high relative humidity conditions due to the MCC influence on observed equilibrium moisture content (figure 5).
See the full technical brochure on “MicroceLac® 100” here
(click the picture to download the brochure)
Benefits MicroceLac® 100
- Excellent compactability and flowability
- Ideal for poorly compactable APIs, e.g. herbal extracts
- Ideal tablet surface for straight forward and economical coating
- High adherence capacity prevents segregation and subsequently improves content uniformity
Source: MEGGLE technical brochure “MicroceLac® 100”
See the overview video of the MEGGLE Dry Powder Inhalation product range here:
Company: MEGGLE is one of the world´s leading manufacturers of pharmaceutical grade lactose and co-processed excipients with expertise of more than 70 years. We encounter lactose in so many areas of our life – reason enough to take a closer look at this multi-functional “white powder”.
Do you need more information or a sample of MicroceLac® 100 excipients?


