Mirtazapine loaded polymeric micelles for rapid release tablet: A novel formulation – In vitro and in vivo studies
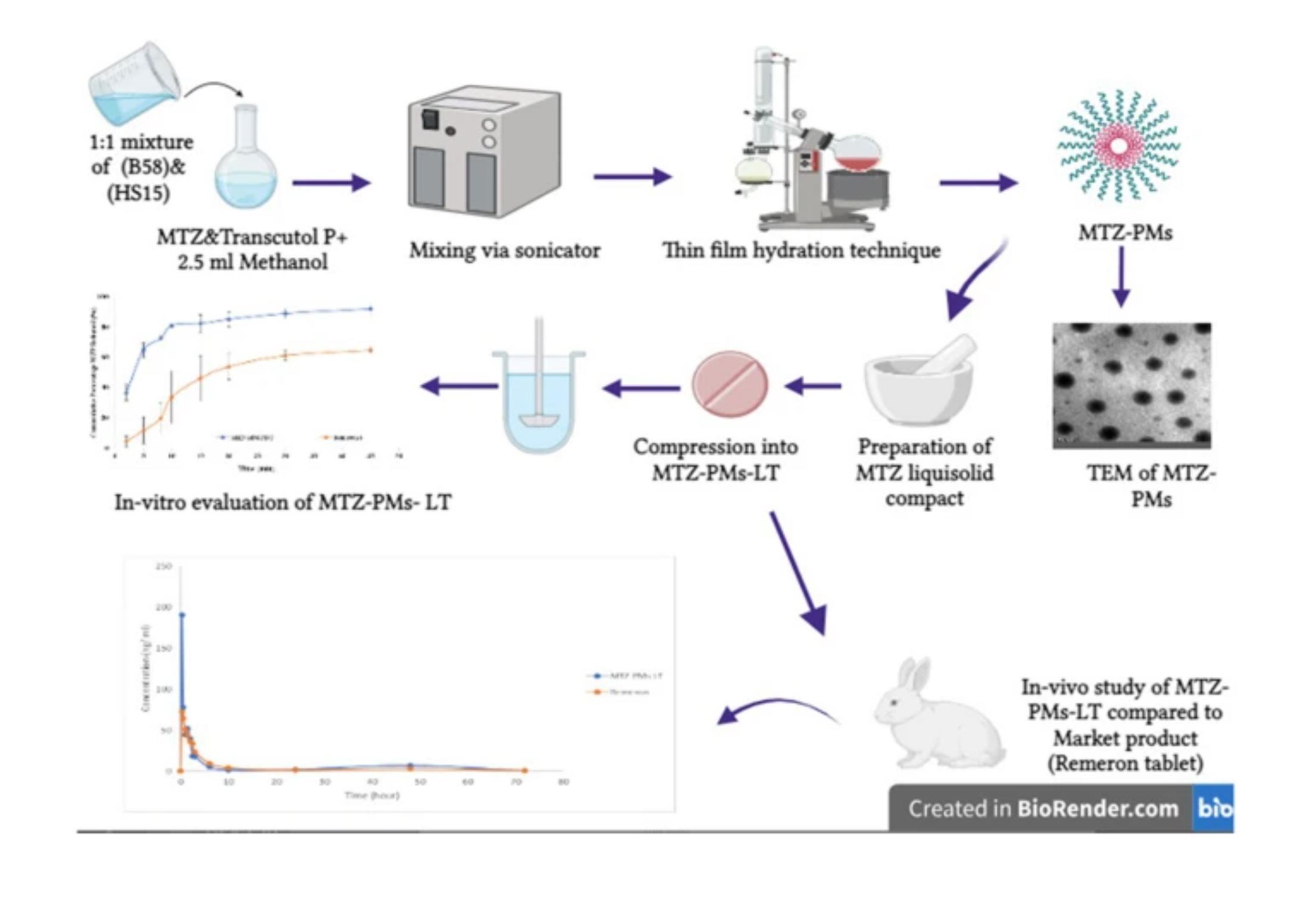
Major depression is a prevalent disorder characterized by sadness, lack of interest or pleasure, interrupted sleep or food, and impaired concentration. Mirtazapine (MTZ), a tetracyclic antidepressant drug, is commonly used to treat moderate to severe depression. MTZ is classified as a BCS class II drug that has shown bioavailability of 50% due to extensive first-pass metabolism. The aim of this research is to develop a delivery platform with enhanced solubility and oral bioavailability of MTZ through formulating polymeric micelles modeled in a rapid release tablet. Mirtazapine loaded polymeric micelles (MTZ-PMs) were formulated to enhance the solubility.
Solutol® HS 15 and Brij 58 were used as combined surfactants in a ratio of (20:1) to MTZ in addition to Transcutol® P as a penetration enhancer. The following in vitro tests were performed: particle size, PDI, zeta potential, solubility factor, stability index, and transmission electron microscopes. Afterward, MTZ-PMs were converted to dry free flowable powder through loading on the adsorptive surface of Aerosil 200; then, the powder mixture was directly compressed (MTZ-PMs-RRT) into 13 mm tablets.
MTZ-PMs-RRT was further investigated using in vitro evaluation tests of the tablets, namely, weight variation, thickness, diameter, hardness, friability, disintegration time, drug content, and in vitro dissolution test, which complied with the pharmacopeial limits. The pharmacokinetic parameters of MTZ-PMs-RRT compared to Remeron® tablet were further investigated in rabbits. The results showed enhanced solubility of MTZ with improved percentage relative bioavailability to 153%. The formulation of MTZ in the form of MTZ-PMs-RRT successfully improved the solubility, stability, and bioavailability of MTZ using a simple and scalable manufacturing process.
Download the full article as PDF here Mirtazapine loaded polymeric micelles for rapid release tablet
or read it here
Materials
Mirtazapine was purchased from Sinochem, China. Solutol (HS15) and BrijVR 58 (B58) (polyethylene glycol hexadecyl ether) were obtained from Sigma-Aldrich Inc., USA. Transcutol P (Trc) (diethylene glycol monoethyl ether) was supplied as a gift by Gattefosse, France. Mannitol was obtained from Shandong Bangye Co. Ltd., China. Explotab (sodium starch glycolate) was obtained from JRS Pharma LP., USA. Magnesium stearate was obtained from Aceto-Corp, USA. Aerosil 200 (colloidal silicon dioxide) was purchased from Degussa Ltd., Germany. Methanol (95%), disodium phosphate hydrogen, and potassium dihydrogen phosphate were obtained from El-Nasr Pharmaceutical Chemicals Co., Egypt. All other solvents and chemicals were of analytical grade. The water used was distilled, deionized water.
El-Helaly, S.N., Rashad, A.A. Mirtazapine loaded polymeric micelles for rapid release tablet: A novel formulation—In vitro and in vivo studies. Drug Deliv. and Transl. Res. (2024). https://doi.org/10.1007/s13346-024-01525-w
Read also our introduction article on Magnesium Stearate here:


