Nanocarrier Vaccines for SARS-CoV-2
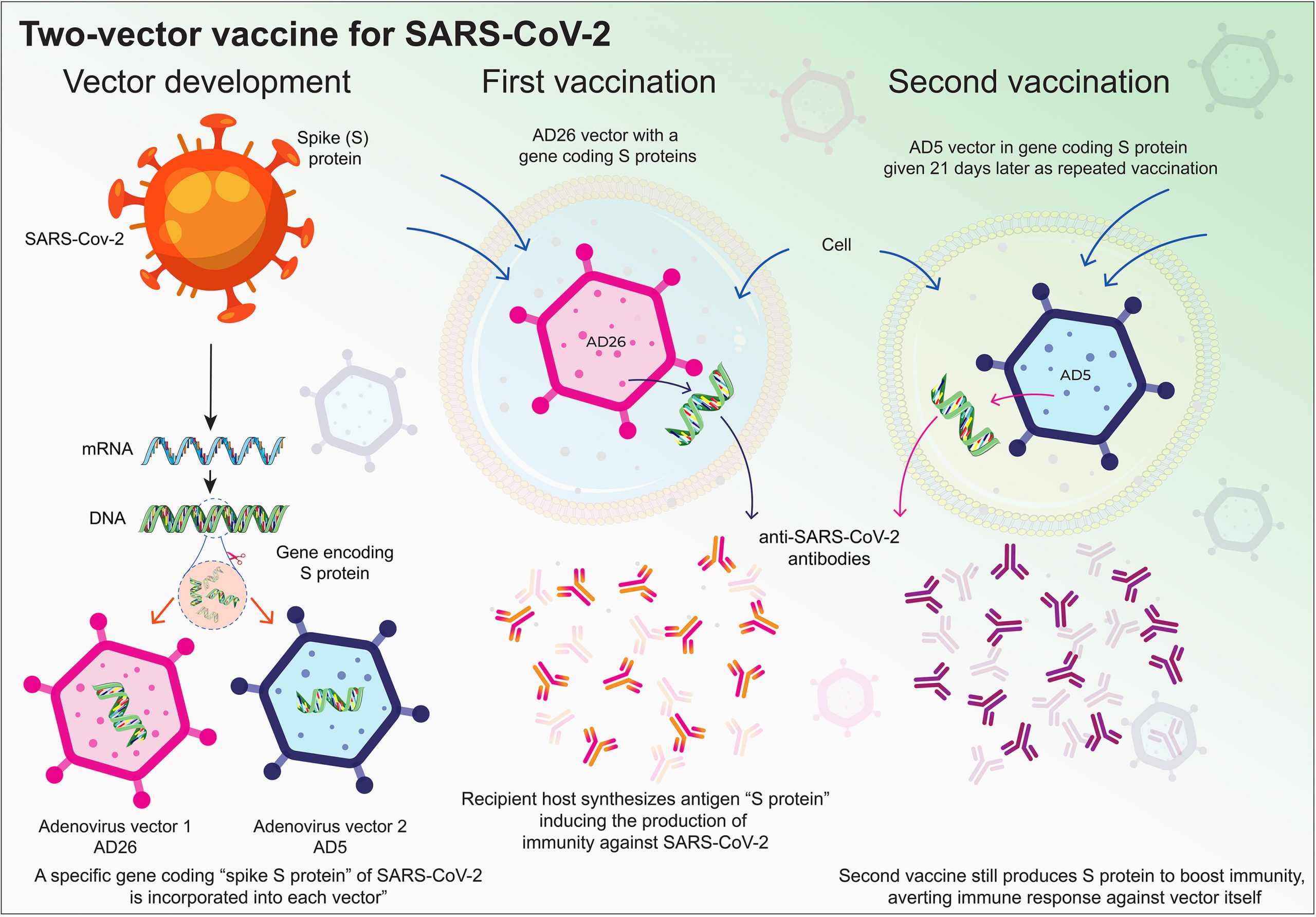
The SARS-CoV-2 global pandemic has seen rapid spread, disease mobidities and death associated with substantive social, economic and societal impact. Treatments rely on re-purposed antivirals and immune modulatory agents focusing on attenuating the acute respiratory distress syndrome. No curative therapies exist. Vaccines remain the best hope for disease control and elimination and the principal global effort with success now being realized. Herein, we summarize those developments with a focus on the role played by nanocarrier delivery.
Overview: Pathways towards an effective COVID-19 vaccine
In late 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) first emerged and on March 11, 2020 it was declared a pandemic [1]. Clinical outcomes ranged from asymptomatic infection to severe acute respiratory distress syndrome (ARDS) and death. The World Health Organization (WHO) named the resultant disease complex coronavirus disease 2019 (COVID-19) [2,3]. COVID-19 has negatively impacted the global socioeconomic well-being of the world’s population. Global lack of health care, infrastructure, and preparedness has intensified the pandemic’s impact [4].
Viral detection, mobilization and control of person-to-person spread served as the primary means for containment. Induction of effective host antiviral immunity against SARS-CoV-2 comes secondary to infection [5,6]. An uncontrolled innate immune response is the signature of virus-induced pro-inflammatory responses for an ARDS. Alveolar macrophage inflammation disrupts cell and tissue homeostasis leading to end-organ lung disease [7,8]. In the absence of a vaccine, virus-induction of adaptive humoral immune responses can attenuate disease progression [9].
A vaccine can elicit protective antiviral responses against SARS-CoV-2. Short of containment it is the most effective means to prevent infection in susceptible people [10]. To this end, there are more than 137 vaccine candidates in development and 23 in Phase 2 or 3 trials [11,12]. One promising candidate BNT162b2 is already approved while several others are soon to be approved for prevention in the United States of America (USA) [13,14]. However, how long an induced immune response remains effective is in question. Final outcomes will depend, in part, on the continuance of a neutralizing antibody response, the limitations seen on viral mutations and the long-term induction of antiviral memory T cell responses [15,16]. The long term efficacy of a SARS-CoV-2 vaccine requires limited boosting and divergent geographic, co-morbid and age-associated population efficacy [17]. Efficacy is defined as long-term prevention of viral infection and in halting transmission in broad populations [18].
Download the full article as a PDF here or read it here
Article Information: Jatin Machhi, Farah Shahjin, Srijanee Das, Milankumar Patel, Mai Mohamed Abdelmoaty, Jacob D. Cohen, Preet Amol Singh, Ashish Baldi, Neha Bajwa, Raj Kumar, Lalit K. Vora, Tapan A. Patel, Maxim D. Oleynikov, Dhruvkumar Soni, Pravin Yeapuri, Insiya Mukadam, Rajashree Chakraborty, Caroline G. Saksena, Jonathan Herskovitz, Mahmudul Hasan, David Oupicky, Suvarthi Das, Ryan F. Donnelly, Kenneth S. Hettie, Linda Chang, Howard E. Gendelman, Bhavesh D. Kevadiya. Nanocarrier Vaccines for SARS-CoV-2, Advanced Drug Delivery Reviews, 2021. https://doi.org/10.1016/j.addr.2021.01.002.

