Nanocrystal-chitosan particles for intra-articular delivery of disease-modifying osteoarthritis drugs
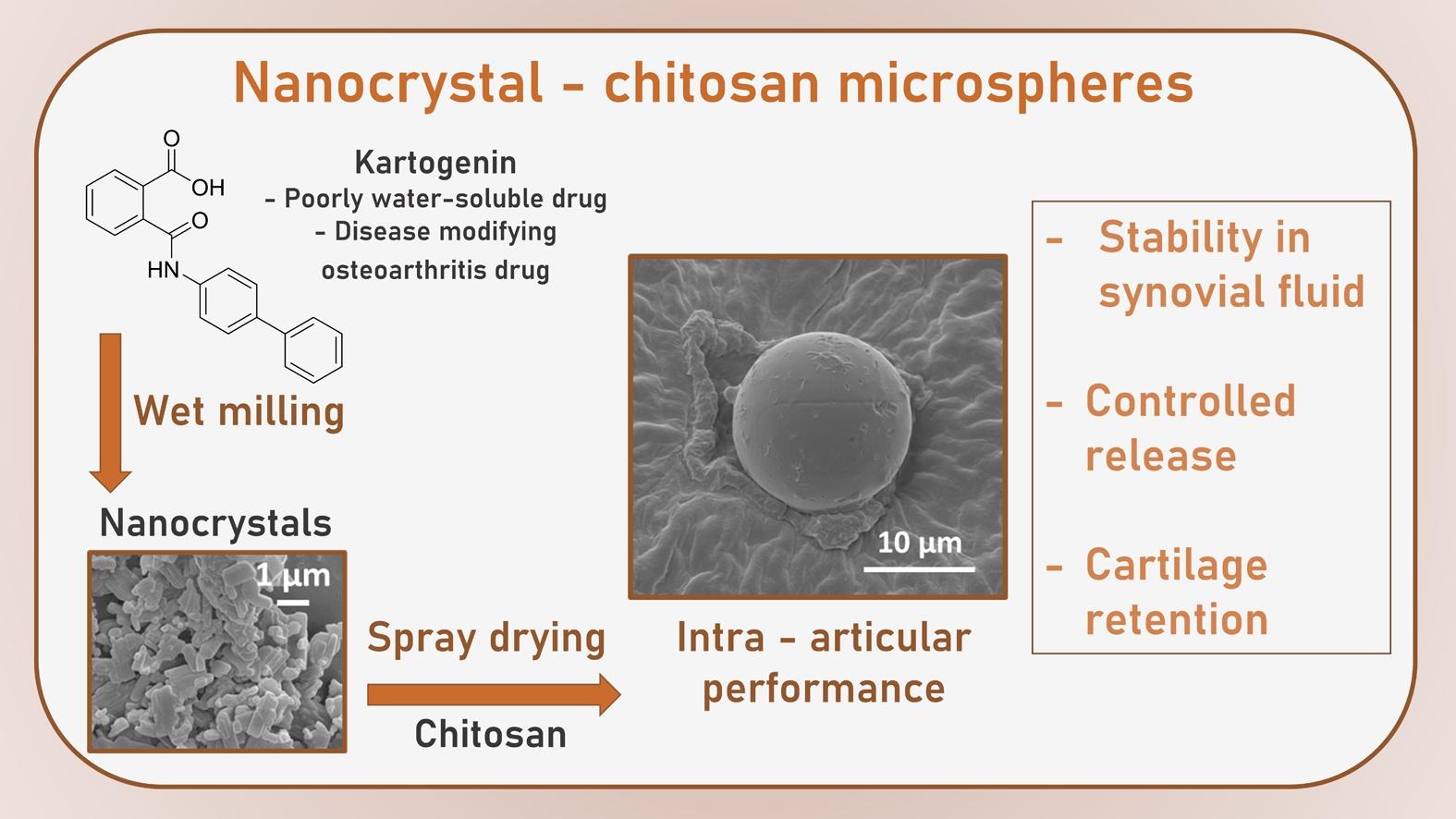
Osteoarthritis is the most common chronic joint disease and a major health care concern due to the lack of efficient treatments. This is mainly related to the local and degenerative nature of this disease. Kartogenin was recently reported as a disease-modifying osteoarthritis drug that promotes cartilage repair, but its therapeutic effect is impeded by its very low solubility. Therefore, we designed a unique nanocrystal-chitosan particle intra-articular delivery system for osteoarthritis treatment that merges the following formulation techniques: nanosize reduction of a drug by wet milling and spray drying. The intermediate formulation (kartogenin nanocrystals) increased the solubility and dissolution rates of kartogenin.
Highlights
- Combined technological approach for poorly-water soluble drugs: wet milling + spray drying.
- Ready-to-use nanocrystal-microsphere powder, compatible with an intra-articular administration.
- Intra-articular delivery of a disease-modifying osteoarthritis drug (kartogenin).
- Stability in synovial fluid and non-toxic in human synoviocytes.
- Cationic chitosan microspheres improve controlled release and kartogenin cartilage retention.
The final drug delivery system consisted of an easily resuspendable and ready-to-use microsphere powder for intra-articular injection. Positively charged chitosan microspheres with a median size of approximately 10 µm acted as a mothership drug delivery system for kartogenin nanocrystals in a simulated intra-articular injection. The microspheres showed suitable stability and a controlled release profile in synovial fluid and were nontoxic in human synoviocytes. The cartilage retention skills of the microspheres were also explored ex vivo using cartilage. This drug delivery system shows promise for advancement to preclinical stages in osteoarthritis therapy and scale-up production.
Download the full article as PDF here: Nanocrystal-chitosan particles for intra-articular delivery of disease-modifying osteoarthritis drugs
or read it here
Materials
Ethanol and tetrahydrofuran were acquired from Fisher Scientific (Waltham, MA, USA). Phthalic anhydride was purchased from Acros Organics (Geel, Belgium), ethyl acetate, glutaraldehyde, dimethylsulfoxide, D-α-Tocopherol polyethylene glycol 1000 succinate, acetonitrile (analytical grade), phosphate buffer saline tablets, polyvinylpyrrolidone (MW 40,000), kartogenin (commercial), 4-aminobiphenyl, low (50–190 kDa), medium (190–310 kDa) and high (310–375 kDa) MW 75–85 % deacetylated chitosan, sterile phosphate buffer saline (PBS), RPMI-1640 and M199 cell media were supplied by Sigma-Aldrich (Sant Louis, MO, USA). Formic acid was acquired from Reactolab SA (Servion, Switzerland). Yttrium-stabilized zirconium oxide (ZrO2) Ø 0.5-mm grinding beads were purchased from Next Advance (Troy, NY, USA). WST-1 cell proliferation assay was purchased from Roche Applied Science (Basel, Switzerland). Fetal bovine serum was purchased from Eurobio (Les Ulis, France). Trypsin-EDTA and penicillin/streptomycin were provided by Gibco® (Invitrogen Inc., Carlsbad, USA). Equine synovial fluid was aspirated from six fetlock joints, obtained after slaughter from healthy adult horses (Communal slaughterhouse, Delémont, Switzerland) and stored at −80 °C. Fresh bovine knee joint cartilage was donated from a local butcher shop (Boucherie Chevaline du Vieux Carouge, Geneva, Switzerland).
Luca Morici, Paula Gonzalez-Fernandez, Sébastien Jenni, Alexandre Porcello, Eric Allémann, Olivier Jordan, Carlos Rodríguez-Nogales, Nanocrystal-chitosan particles for intra-articular delivery of disease-modifying osteoarthritis drugs,
International Journal of Pharmaceutics, Volume 651, 2024, 123754, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.123754.

