Development of nanoemulsion loaded acyclovir nanogel for transdermal delivery and its evaluation
Abstract
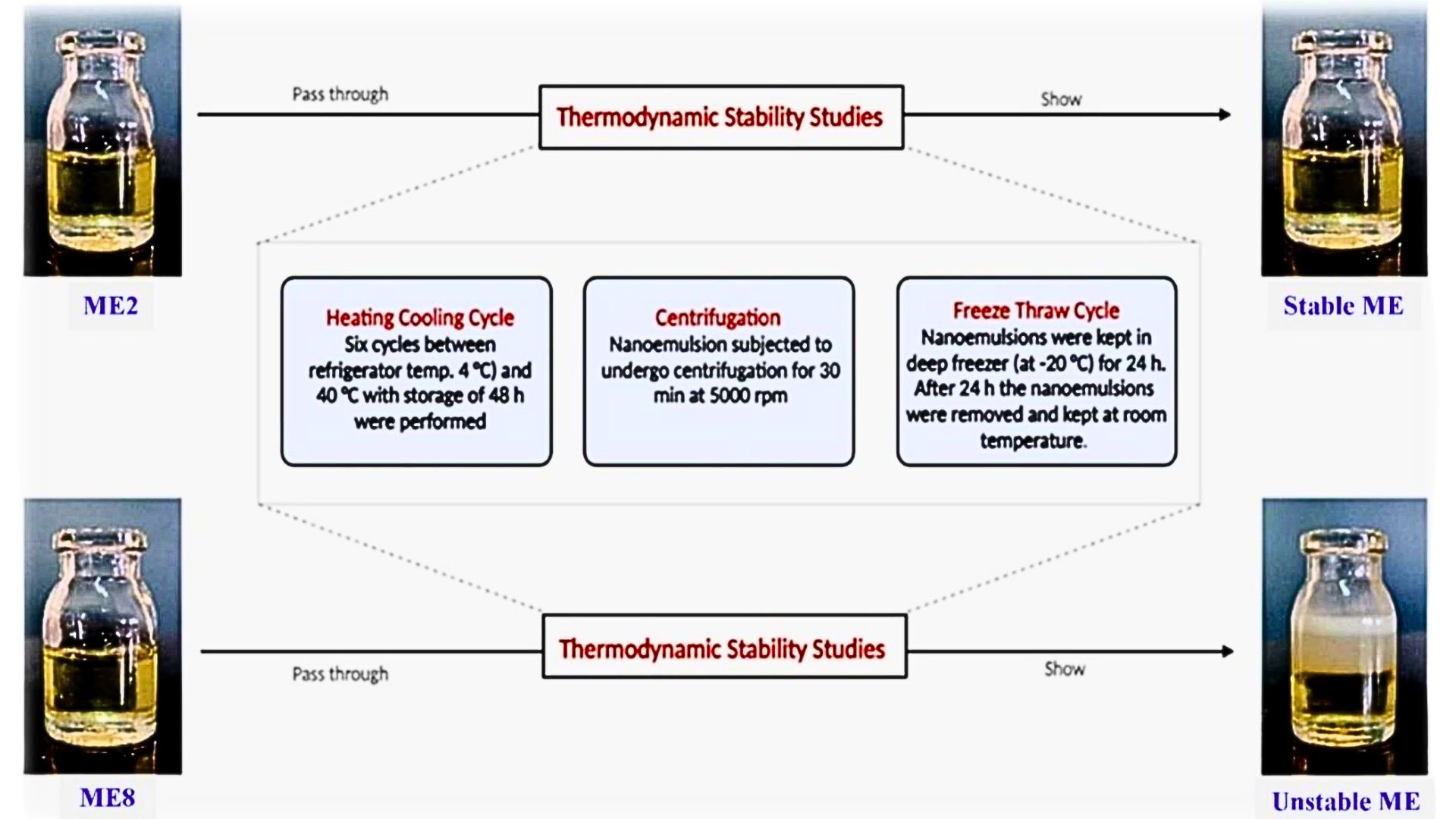
Acyclovir (ACV) is an antiviral drug that is primarily used to treat herpes zoster virus (HSV) infections. HSV infections in deeper regions of the skin, i.e., nerve junctions, and appeared as a red spot on the upper part of the skin. The objective of the research is to formulate and evaluate an emulgel loaded with ACV microemulsion (ME) in order to treat herpes infections and to get effects from the deeper region of the skin. MEs were prepared by high-pressure homogenization, and then microemulgels were prepared by the dispersion method. Microemulgel characteristics are being determined, and an in vitro diffusion study was performed. In this study, we have developed and evaluated an ACV-loaded microemulgel using Carbopol 940 as the gelling agent. The characteristics and stability of ACV-loaded microemulgels were studied, and in vitro drug diffusion of the optimized microemulgel, i.e., MEG3, was found to be 95±2.4% with a sustained effect for 8 hours. Observation shows that the optimized microemulgel has a smaller globule size, and hence it may move to the deeper regions of the skin. Stability studies did not show any significant change; hence, it is apparent that the drug substance or product will remain within the acceptance criteria during shelf life. It was concluded that the ACV-loaded microemulgel was prepared successfully for transdermal delivery.
Introduction
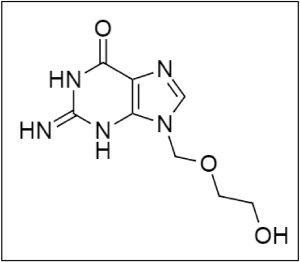
Acyclovir (ACV) is a class of antiviral medications called synthetic nucleoside analogues that mimic guanosine, which is defined by its structure (Figure 1). It is a first-line drug used to treat infections caused byHSV. ACV was approved by the Food and Drug Administration (FDA) to treat HSV encephalitis and genital herpes1. Some other non-FDA approved indications are herpes zoster, varicella zoster, and mucocutaneous HSV, in which ACV can be used. It prevents the synthesis of viral agents by incorporating itself into the viral DNA2 . The bioavailability of ACV is about 10 20%, which is considered to be much less, and the absorption through the topical route is not more than 10%.
Therefore, in the current study, ACV is being used to get effective therapy, reduced side effects, and also reduced the dose of ACV through transdermal delivery3. In the transdermal delivery system (TDDS), bioactive ingredients are delivered across the skin to the systemic circulation. The application of drugs to the skin is considered to be one of the most important target sites4-6. It also provides controlled absorption, more uniform plasma levels, reduced side effects, painless and simple application, and the flexibility of terminating drug administration by simply withdrawing it from the skin7-8. Microemulgels are dosage forms that have the properties of microemulsions as well as emulgels9-11. The prepared formulations are known as microemulgels when both microemulsion and gel are combined in the dosage form12-13. They offer a significant surface area for drug absorption, and the oil portion promotes bioavailability by enhancing drug permeability.
Incorporating microemulsion into gel also increases its stability14-16. Microemulgel will be a more significant dosage form for the delivery of drugs through the transdermal route. The current research is to prepare ACV-loaded microemulgels, which are considered to help reduce the side effects caused by the ACV. The formulated microemulgel is evaluated to determine its consistency, drug diffusion, viscosity, and stability to provide an effective therapy for HSV.
Download the full article as PDF here: Development of nanoemulsion loaded acyclovir nanogel for transdermal delivery and its evaluation
Materials
ACV was purchased from Mangalam Drugs & Organic Ltd., Mumbai; isopropyl myristate (IPM), Tween 80, polyethylene glycol 400 (PEG 400), ethanol, Carbopol 940, glycerine, methyl paraben, propyl paraben, triethanolamine, sodium chloride, potassium chloride, sodium phosphate dibasic, potassium phosphate monobasic, hydrochloric acid (HCl), and isopropyl alcohol were purchased from the Research-Lab Fine Chem Industries, Mumbai. The diffusion membrane was purchased from Dolphin Pharmacy Instruments Pvt. Ltd, Mumbai.
Tejas Suresh Telange, Shivani Santosh Pawar, Akash Jayendra Amkar, Bhushan Rajendra Rane, Ashish Suresh Jain, Development of nanoemulsion loaded acyclovir nanogel for transdermal delivery and its evaluation, Pharm Sci Asia 2023; 50(4), 347-360, Shri D. D. Vispute College of Pharmacy and Research Center Panvel, Maharshtra, India, DOI:10.29090/psa.2023.04.23.616

