Research on classification of natural plant products and critical factors affecting tablet disintegration properties based on texture analyzer
Abstract
The complex physical properties of natural plant products (NPPs) greatly influence the disintegration of NPP tablets, and the evaluation of their disintegration properties is generally overlooked. To solve this problem, the study aimed to reveal the disintegration behavior of NPP tablets by the stress relaxation method of texture analyzer and investigate the key attributes affecting the disintegration of NPPs. 24 types of NPPs were systematically evaluated and divided into 3 kinds of groups: expansive NPPs, dissolved NPPs, and disintegrating NPPs. The disintegration characteristics of each group were significantly different. 9 kinds of characteristic parameters of disintegration kinetics of NPP tablets were used to analyze in detail. The results of Pearson’s correlation coefficient showed that there was a strong correlation between the fundamental properties of powders and the disintegration kinetics of tablets. The results of principal component analysis (PCA) confirmed the validity of the classification method. Additionally, the medicament portions and texture characteristics of NPPs are the key factors influencing tablet disintegration. The disintegration of NPPs may also be affected by the dissolution. Thus, the disintegration behavior of NPP tablets was investigated and the factors influencing disintegration were revealed.
Introduction
Tablets are one of the most popular oral solid dosage forms because they have the advantages of convenient take, accurate dosing, storage stability, and ease of manufacturing [1], [2]. Tablets usually undergo disintegration and dissolution before being absorbed in the gastrointestinal tract. The disintegration process of break-up of powder compacts into small particles facilitates to increase the surface area of the particles, which is essential for drug dissolution and improved bioavailability [3], [4].
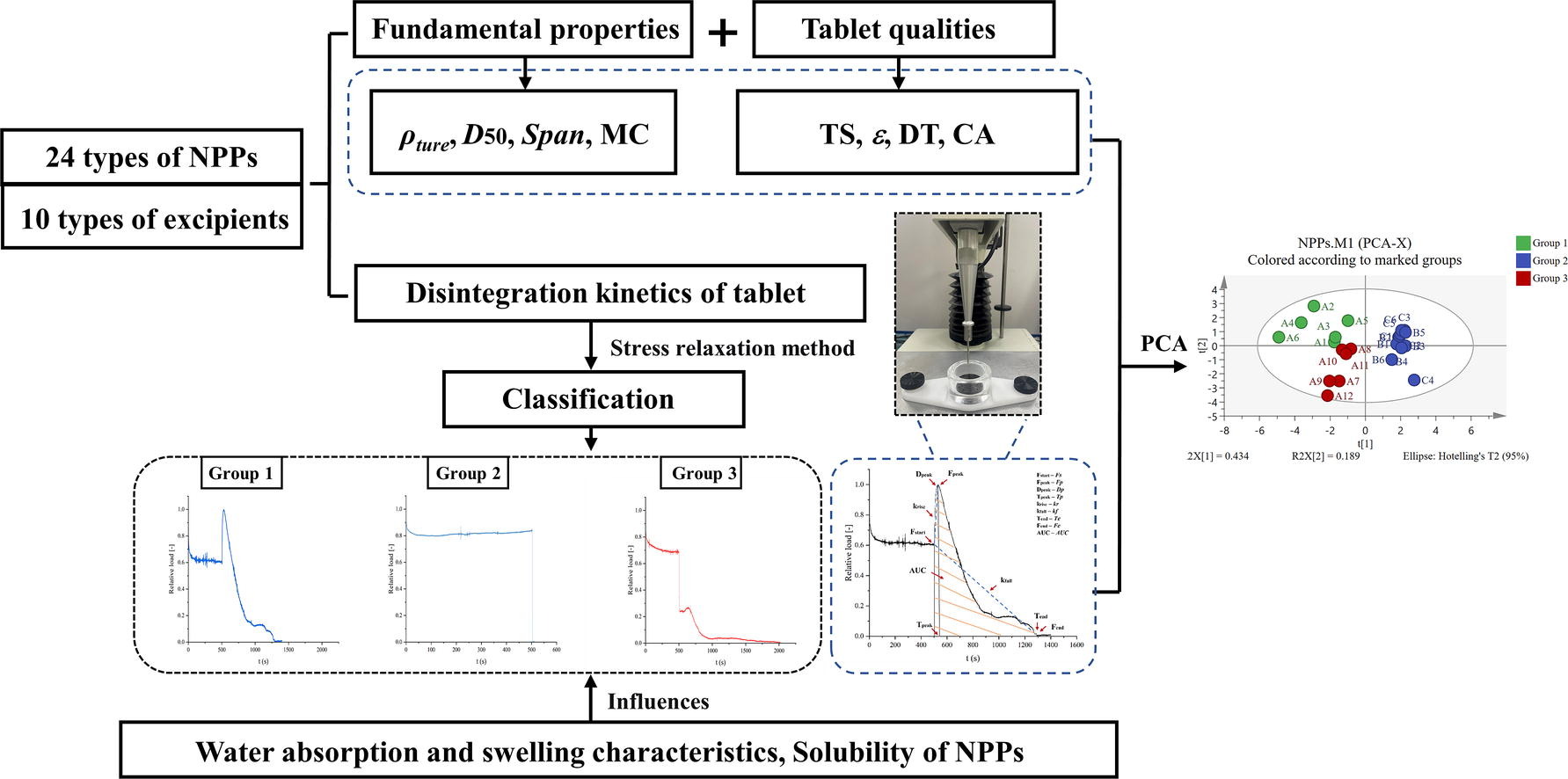
Wicking, considered a prerequisite for initiating disintegration, is the process by which liquid permeates in the pores of the tablet through capillary action. The wicking reflects the rate of liquid uptake by the tablet. With the penetration of the liquid, the particles in the tablet expand, the pores are constantly filled, and the force is exerted on the surrounding particles. When this force exceeds the inter-particle bonding forces, disintegration occurs [5]. Therefore, expansion is an important mechanism of disintegration, which includes swelling and shape recovery. The swelling is manifested as the three-dimensional volume expansion of the disintegrating particles, while the shape recovery process is one-dimensional, opposite to the direction of the applied force of tablet compression [6]. In addition, disintegration may also be the result of dissolution of soluble components in the tablets. Studies have shown that the fundamental properties (particle size, solubility, wettability, etc.) of active pharmaceutical ingredients (APIs)/excipients powder have a significant impact on the liquid absorption and disintegration process of tablets [7].
The disintegration test lacks specific information about the disintegration process of tablets, and cannot fully reveal the disintegration mechanism. Consequently, it is urgent to carry out studies on the relationship between liquid absorption properties, swelling behavior, and disintegration kinetics to gain a full understanding of the whole disintegration process and potential mechanism of tablets. Based on process analysis technology (PAT), sophisticated and advanced evaluation methods were used to study each stage of the disintegration process and its microstructure, which can effectively solve the shortcomings of conventional methods. Researchers have developed experimental devices to characterize liquid uptake and swelling behavior [8], [9]. The application of spectral imaging and optical imaging technology makes the microstructure of disintegrating particles visualized [10], [11], [12]. Additionally, variations in the mechanical properties of tablets during the disintegration process can be obtained by the texture analyzer [13], [14]. Statistical analysis of data and the application of machine learning have become effective tools to evaluate APIs/ excipients material properties on tablet quality attributes [15].
Natural plant products (NPPs) have attracted much attention due to their importance in preventing and treating various diseases, as well as the advantages of multiple ingredients and relatively few side effects [16], [17]. Superior disintegration performance can make NPP tablets show efficient and rapid activity, which is important for oral disintegrating tablets and NPP dispersing tablets [18]. Notably, the fundamental properties of NPP may affect the disintegration and dissolution of tablets. For example, NPP powders mainly obtained by extracting liquid are soluble, and studies have shown that the solubility of soluble components can affect both wicking and disintegration, especially in tablets containing high concentrations of soluble components [3].
In fact, high loading of API is common in NPP tablets [19]. Therefore, it is necessary to systematically understand the contribution of NPP powders to the disintegration of tablets. Despite the increasing number of studies on the tablet disintegration potential mechanism, as well as influence, have been found in tablets composed of chemicals [20], [21], some results are not particularly applicable to NPPs. The main reasons may be: 1) NPPs are composed of more diverse and complex components than chemical drugs; 2) A bulk of excipients in the formulation have certain characteristics, and the combination with APIs adds variability to the complex process of tablet disintegration; 3) The process parameters (such as direct tablet compaction, wet or dry granulation, and compaction conditions) in the manufacturing of NPP tablets had significant effects on the disintegration; 4) the sensitivity of NPPs to storage conditions (such as moisture and temperature) and media. Therefore, owing to the complex physical properties of NPPs, the development of NPP tablets disintegration is constrained. In particular, the evaluation of disintegration characteristics of NPP tablets is generally overlooked due to a lack of systematic research. The classification of NPPs based on disintegration characteristics is rarely reported.
In light of the above, the study aimed to reveal the disintegration behavior of NPP tablets by the stress relaxation measurement of texture analyzer, divide NPP tablets into several groups with different disintegration mechanisms, and investigate the contribution of key attributes affecting the disintegration performance of each group of NPPs. 24 kinds of commonly used NPP powders were selected as model materials, including 6 kinds of ethylalcohol extraction (E-E), 6 kinds of water extraction (W-E), and 12 kinds of direct pulverization (D-P). Meanwhile, 10 kinds of commonly used excipients with expansion characteristics were selected for comparison. Firstly, the fundamental properties of NPP powders and the quality characteristics of NPP tablets were characterized. Next, the stress relaxation measurement by texture analyzer was used to evaluate the disintegration behavior of NPP tablets, and NPPs were classified according to the disintegration kinetics of tablets. Then, Pearson’s correlation coefficient showed the correlation between the fundamental properties of the powders and the parameters of the disintegration kinetics of tablets, and principal component analysis (PCA) was utilized to verify the classification method. Finally, the crucial factors that influence the disintegration of NPP tablets were investigated by evaluating the water absorption and swelling characteristics and changing the disintegrating medium environment.
Read more on classification of natural plant products and critical factors affecting tablet disintegration properties
Zhenda Liu, Chuting Shi, Ying Fang, Liangfeng Wang, Lijie Zhao, Lan Shen, Research on classification of natural plant products and critical factors affecting tablet disintegration properties based on texture analyzer, Advanced Powder Technology, Volume 35, Issue 3, 2024, 104347, ISSN 0921-8831, https://doi.org/10.1016/j.apt.2024.104347.
Read more on Disintegrants – Pharmaceutical Excipients here:


