Disintegrants – Pharmaceutical Excipients
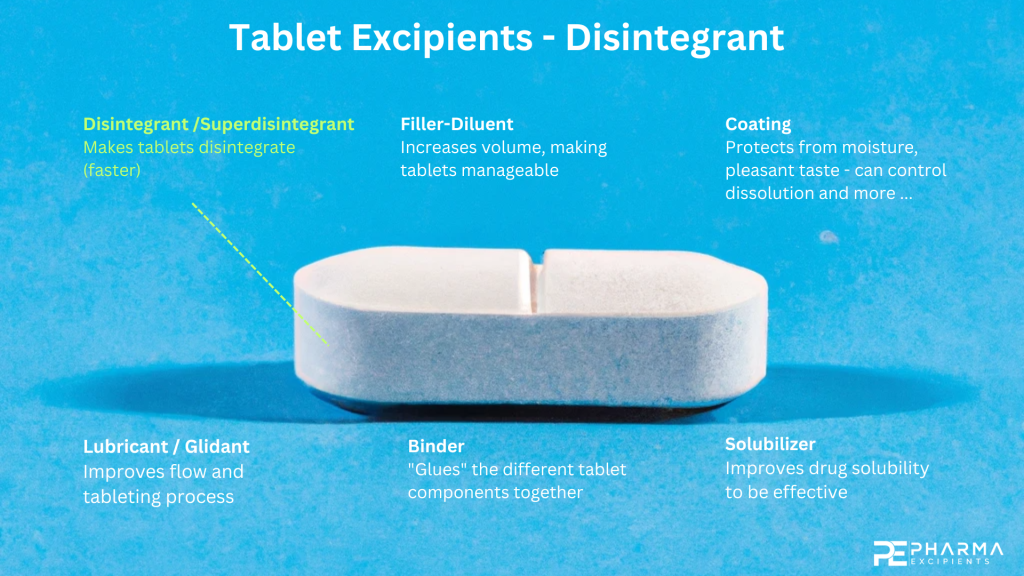
Disintegrants and superdisintegrant excipients are essential components used in the pharmaceutical industry to enhance the dissolution and bioavailability of active pharmaceutical ingredients (APIs). These excipients facilitate the disintegration and rapid breakup of tablets or capsules, which ultimately increases their rate of absorption in the body.
Listen to the article:
Definition of Disintegrants and Superdisintegrants
According to the United States Pharmacopeia (USP/NF), disintegrants are defined as “agents added to tablet or capsule formulations to facilitate the breakup or disintegration of the tablet or capsule into smaller particles that dissolve more rapidly in the gastrointestinal fluids” (USP 41-NF 36 General Chapter <701> Disintegration). On the other hand, superdisintegrants are defined as “highly effective disintegrants that, when used at low levels, provide rapid disintegration and dissolution of the tablet or capsule dosage form” (USP 41-NF 36 General Chapter <2040> Superdisintegrants).
The European Pharmacopoeia (Pharm Eur) defines disintegrants and superdisintegrants in a similar manner, but also includes that these agents should be non-toxic, non-irritant, and compatible with other components of the dosage form.
Definition of disintegrants by Peter C. Schmidt in “Pharmazeutische Hilfsstoffe” (1) Disintegrants are substances that are intended to promote the rapid disintegration of a tablet. The mechanism of disintegration can be described as follows: during disintegration, the tablet absorbs water capillary in the disintegration medium. The penetrating water reaches the particles of the disintegrating agent embedded between the granules – or the fillers and binders in the case of direct tableting – in the tablet matrix, which spontaneously absorb water under swelling without dissolving. This results in the so-called swelling pressure, which is the driving force of tablet disintegration (List and Muazzam1979a and 1979b). The amount of swelling pressure depends on the material of the disintegrant and also on its particle size. The particles must be large enough to completely fill the space between the granules or the fillers and binders in order to generate a high swelling pressure. Micronized disintegration aids generally do not promote disintegration.

Schmidt (1) makes the following differentiation of disintegrants by origin. Many have other functions such as binder and fillers.
-
Natural Disintegrants
- Alginic acid
- Bentonit
- Microcrystalline Cellulose
- Powdered Cellulose
- Guargalactomannan
-
Semi-Synthetic Disintegrants
- Carboxymethylcellulose Calcium
- Carmellose Sodium
- Croscarmellose Sodium
- Sodium Starch Glycolate Type A, B
- Low-Substituted Carboxymethylcellulose Sodium
- Low-Substituted Hydroxypropyl Cellulose
-
Synthetic Disintegrants
- Crospovidon
NEWS ON PHARMAEXCIPIENTS “DISINTEGRANT / SUPERDISINTEGRANT”
Excipients Used as Disintegrants and Superdisintegrants
There are several excipients used as disintegrants and superdisintegrants in the pharmaceutical industry, including:
- Starches: This is the most commonly used disintegrant in the industry. It includes corn starch, potato starch, and modified starches such as pregelatinized starch, sodium starch glycolate, and starch 1500.
- Cellulose-based excipients: These include microcrystalline cellulose, croscarmellose sodium, sodium carboxymethyl cellulose, and hydroxypropyl methylcellulose.
- Natural gums: These include guar gum, xanthan gum, and locust bean gum.
- Ion exchange resins: These include polacrilin potassium and Amberlite IRP69.
- Calcium silicates: These include dicalcium phosphate and tricalcium phosphate.
- Others: These include sodium alginate, cross-linked polyvinylpyrrolidone, and chitosan.
Chemical Background of Disintegrants and Superdisintegrants
The chemical structure of disintegrants and superdisintegrants varies widely depending on the excipient used. Starches, for example, are polysaccharides made up of glucose molecules linked together by alpha 1-4 glycosidic bonds. Modified starches have been chemically modified to improve their functionality. Sodium starch glycolate, for instance, is a cross-linked sodium carboxymethyl ether of starch, while starch 1500 is a pre-gelatinized maize starch that has been modified with sodium sulfate.
Cellulose-based excipients are also polysaccharides, but they are made up of glucose molecules linked together by beta 1-4 glycosidic bonds. Microcrystalline cellulose, for example, is a partially depolymerized cellulose that has been mechanically processed to produce small, crystalline particles. Croscarmellose sodium, on the other hand, is a cross-linked sodium carboxymethyl cellulose.
Natural gums, such as guar gum, xanthan gum, and locust bean gum, are polysaccharides that are obtained from plant sources. They are long chains of sugar molecules linked together by glycosidic bonds. These gums have the ability to absorb water and swell, which helps to facilitate the breakup of the tablet or capsule.
Ion exchange resins, such as polacrilin potassium and Amberlite IRP69, are synthetic polymers that contain functional groups that can exchange ions. They work by absorbing water and swelling, which disrupts the tablet or capsule structure and promotes rapid disintegration.
Calcium silicates, such as dicalcium phosphate and tricalcium phosphate, are inorganic compounds that are commonly used as excipients in the pharmaceutical industry. They have the ability to absorb water and swell, which helps to facilitate disintegration.
Sodium alginate is a natural polysaccharide obtained from brown algae that has been modified with sodium ions. Cross-linked polyvinylpyrrolidone is a synthetic polymer that has been cross-linked to increase its functionality, while chitosan is a natural polymer derived from chitin.
In conclusion, disintegrants and superdisintegrant excipients are essential components used in the pharmaceutical industry to enhance the dissolution and bioavailability of APIs. There are several excipients used as disintegrants and superdisintegrants, including starches, cellulose-based excipients, natural gums, ion exchange resins, calcium silicates, and others. These excipients have different chemical structures and mechanisms of action, but they all work to facilitate the rapid disintegration of tablets or capsules. The use of disintegrants and superdisintegrants in pharmaceutical formulations is an important factor in improving the effectiveness of drugs and ensuring patient safety.
Disintegrant Excipients on pharmaexcipients.com – in alphabetical order
| Product | Composition | Manufacturer |
| AC-DI-SOL® SD-711 | Croscarmellose Sodium | DuPont |
| CORN STARCH 400L-NF | Native Starches | Roquette |
| DiCom® PL009 P60 | Maize starch, Microcrystalline Cellulose | Gangwal Healthcare |
| EMCOSOY® | Soy Polysaccharides | JRS Pharma |
| EXPLOTAB® | Sodium Starch Glycolate | JRS Pharma |
| GLYCOLYS® | Sodium starch glycolate | Roquette |
| GLYCOLYS® LOW PH | Sodium starch glycolate | Roquette |
| GLYCOLYS® Low Solvent | Sodium starch glycolate | Roquette |
| GLYCOLYS® LV | Sodium starch glycolate | Roquette |
| GLYCOLYS® MAIZE | Sodium starch glycolate | Roquette |
| Kollidon® CL | Crospovidone | BASF |
| Kollidon® CL-SF | Crospovidone | BASF |
| Ludiflash® | Crospovidone, Mannitol, Polyvinyl acetate | BASF |
| Ludipress® LCE | Kollidon® 30, Lactose Monohydrate, Povidone | BASF |
| LYCATAB® C | Pregelatinised Starch | Roquette |
| LYCATAB® C LM | Pregelatinised Starch | Roquette |
| LYCATAB® CT | Pregelatinised Starch | Roquette |
| MAIZE STARCH 5% | Maize starch | Roquette |
| MAIZE STARCH AMYLO N-400 | Maize starch | Roquette |
| MAIZE STARCH B | Maize starch | Roquette |
| MAIZE STARCH EXTRA WHITE | Maize starch | Roquette |
| MAIZE WAXY STARCH N-200 | Maize starch | Roquette |
| MICROCEL™ 101 SD | Microcrystalline Cellulose | Roquette |
| MICROCEL™ 102 SD | Microcrystalline Cellulose | Roquette |
| MICROCEL™ 112 SD | Microcrystalline Cellulose | Roquette |
| MICROCEL™ 200 SD | Microcrystalline Cellulose | Roquette |
| MICROCEL™ 301 SD | Microcrystalline Cellulose | Roquette |
| MICROCEL™ 302 SD | Microcrystalline Cellulose | Roquette |
| MICROCEL™ MC-101 | Microcrystalline Cellulose | Roquette |
| MICROCEL™ MC-102 | Microcrystalline Cellulose | Roquette |
| MICROCEL™ MC-112 | Microcrystalline Cellulose | Roquette |
| MICROCEL™ MC-12 | Microcrystalline Cellulose | Roquette |
| MICROCEL™ MC-200 | Microcrystalline Cellulose | Roquette |
| MICROCEL™ MC-302 | Microcrystalline Cellulose | Roquette |
| Omyanutra® 300 Flash | Calcium Carbonate, Tribasic Calcium Phosphate | Omya |
| Parteck® CCS Croscarmellose Sodium | Croscarmellose Sodium | Merck KGaA |
| PEA STARCH N-735 | Native Starches | Roquette |
| PEARLITOL® FLASH | Mannitol & Maize Starch | Roquette |
| Plasdone K-12 | Crospovidone | Ashland |
| Polyplasdone Crospovidone | Crospovidone | Ashland |
| Polyplasdone INF-10 (Type B) | Crospovidone | Ashland |
| Polyplasdone Ultra (Type A) | Crospovidone | Ashland |
| Polyplasdone Ultra-10 (Type B) | Crospovidone | Ashland |
| Polyplasdone XL-10 (Type B) | Crospovidone | Ashland |
| POTATO STARCH 6% | Potato Starch | Roquette |
| POTATO STARCH 8% | Potato Starch | Roquette |
| POTATO STARCH SUPRA NP | Potato Starch | Roquette |
| Powdered GP-USP | Native Starches | Roquette |
| POWDERED NF CORN STARCH | Corn Starch | Roquette |
| Primojel® | Sodium starch glycolate | DFE PHARMA |
| PVP-P SANAQ® | Crospovidone | Pharmatrans SANAQ |
| SOLUTAB® A | Croscarmellose Sodium | Roquette |
| SOLUTAB® A-IP | Croscarmellose Sodium | Roquette |
| SOLUTAB® EDP | Croscarmellose Sodium | Roquette |
| Solvostar | Sodium starch glycolate | Gangwal Healthcare Private Limited |
| SSG SANAQ® | Sodium starch glycolate | Pharmatrans SANAQ |
| StarLac® | Co-processed lactose starch | Roquette |
| STARLOSE® | Lactose Monohydrate, Maize starch | Gangwal Healthcare |
| VIVAPHARM® PVPP XL | Crospovidone | JRS Pharma |
| VIVAPHARM® PVPP XL-10 | Crospovidone | JRS Pharma |
| VIVASOL ® | Croscarmellose Sodium | JRS Pharma |
| VIVASOL® GF | Croscarmellose Sodium | JRS Pharma |
| VIVASOL® GF LM | Croscarmellose Sodium | JRS Pharma |
| VIVASTAR® P | Sodium Starch Glycolate | JRS Pharma |
| VIVASTAR® P 1000 SF | Sodium Starch Glycolate | JRS Pharma |
| VIVASTAR® P 3500 | Sodium Starch Glycolate | JRS Pharma |
| VIVASTAR® P 5000 | Sodium Starch Glycolate | JRS Pharma |
| WHEAT STARCH TB | Wheat Starch | Roquette |
References
(1) „Pharmazeutische Hilfsstoffe“ Peter C. Schmidt · Siegfried Lang


