Optimisation of the Manufacturing Process of Organic-Solvent-Free Omeprazole Enteric Pellets for the Paediatric Population: Full Factorial Design
Abstract
Liquid formulations are mostly used in the paediatric population. However, with certain active pharmaceutical ingredients (APIs), it is very difficult to guarantee quality and stability; this is the case, for example, with omeprazole. Omeprazole is used as a model drug due to the lack of a paediatric formulation meeting gastro-resistance requirements, which remains a challenge today. In this experimental study, the development of enteric polymer-coated pellets is proposed. It is proposed to use aqueous coating dispersions without the use of organic solvents, which are commonly used in fluidised bed coatings. To do this, the design of experiments method is used as a statistical tool for experiment creation and the subsequent analysis of the responses. In particular, this study uses a randomised full factorial design. The mean weight increases of the protective layer and the enteric coating are chosen as factors. Each factor is assigned two levels. Therefore, the design of the used experiments is a 22 + 1 central point. Overall, the obtained pellets can be an alternative to the compounding formulas of omeprazole that are currently used in the paediatric population, which do not meet the gastro-resistance specifications necessary to guarantee the therapeutic efficacy of this active ingredient.
Introduction
Administering drugs in the paediatric population remains difficult due to the lack of pharmaceutical forms adapted to the needs of paediatric patients. One setting in which this difficulty is evident is hospital pharmacy services, which resort to classical formulation techniques to try to overcome the lack of marketed paediatric medicines. Oral liquid preparations are used as they are the most suitable for such patients; they avoid the need to swallow tablets or capsules and allow simple dosage adjustment according to body weight or body surface area. Generally, these preparations are not subjected to the same quality, safety, and stability tests as marketed medicines and medical devices due to the inability and lack of hospital facility resources to perform these controls. As a result, many medicines are limited with regard to their use in paediatric patients [1,2].
Omeprazole (OME) is a drug with a very evident lack of a paediatric dosage form that meets the stability, safety, and quality requirements demanded by drug regulatory agencies (FDA, EMA, and WHO). OME-compounding formulas used in paediatrics do not conform to their physicochemical, pharmacokinetic, and pharmacodynamic characteristics, and, therefore, the therapeutic effectiveness is directly affected [3,4,5]. OME is a selective and irreversible proton pump inhibitor (PPI) active substance (API). It is widely used in children and adults to treat peptic ulcers, dyspepsia, gastro-oesophageal and laryngopharyngeal reflux, and Zollinger–Ellison syndrome because of its good tolerability and few adverse effects. It is a substituted benzylimidazole derivative in the form of a racemic mixture of two enantiomers. It is a prodrug that is active in acidic media and that reduces acid production in the stomach, relieves symptoms, and promotes gastrointestinal tract healing. Its stability is directly dependent on pH; it remains practically stable in alkaline conditions but degrades rapidly in acidic conditions. It is absorbed in the small intestine; thus, it must be protected from the acidic environment of the stomach. Otherwise, it will not reach its therapeutic target [6,7,8,9,10].
In general, most galenic development is performed non-systematically; to study the effects of a specific factor on a selected response, the levels of each factor are changed separately while keeping all other factors constant. This experimental methodology involves many experiments and is usually influenced by the experience of the researcher and overall is neither an efficient nor cost-effective strategy [11,12]. In this situation, FFD has several advantages over a traditional experimental methodology, such as the provision of the maximum amount of information with the minimum number of experiments, the possibility of increasing the number of factors studied or their levels, and considering possible interactions between factors, simple modelling results, and a thorough search for the optimal response, among others [13,14].
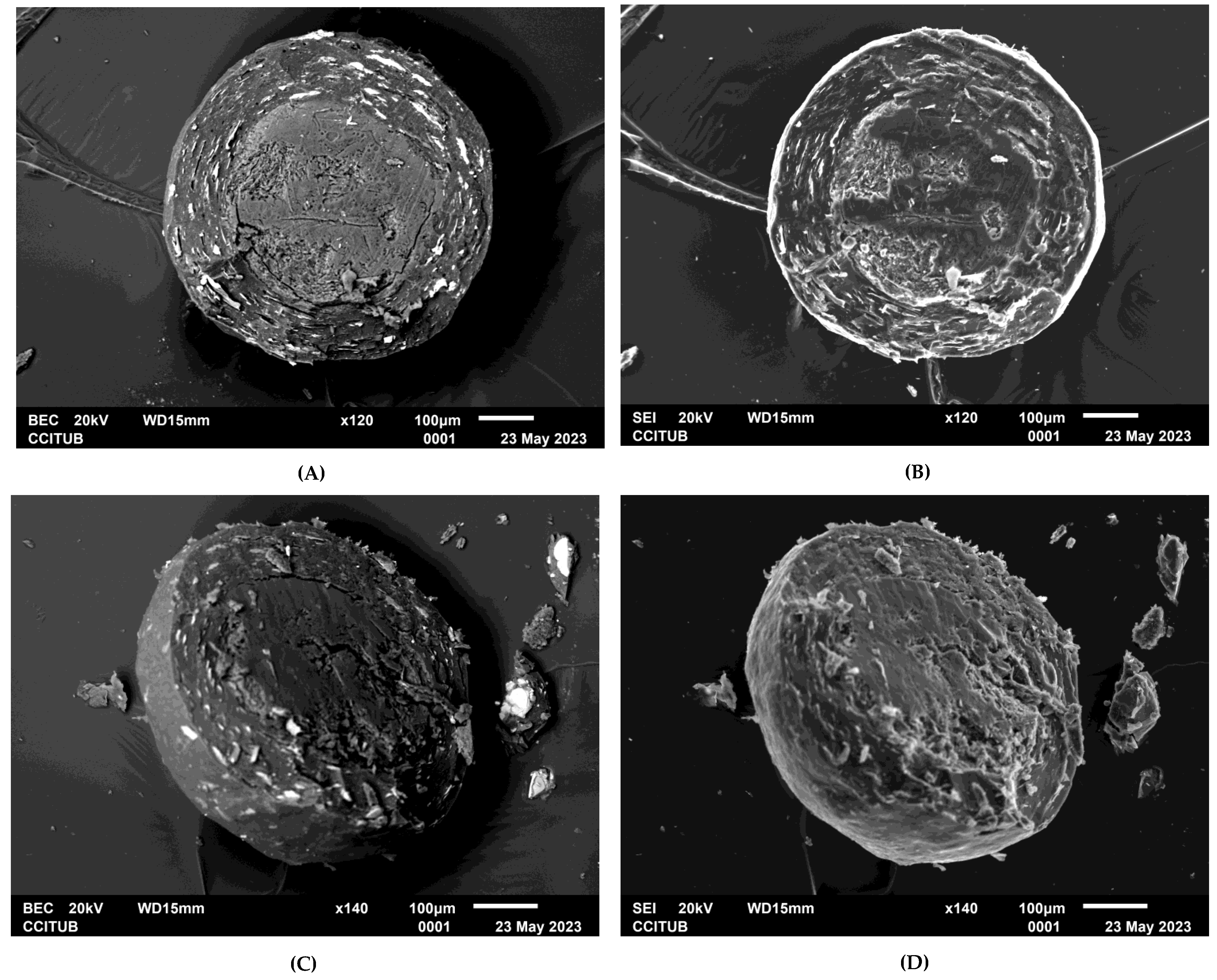
Developing a paediatric dosage form of OME remains a challenge today, as alkaline liquid dispersions are often prepared, which do not ensure the protection of the OME in the gastric environment. Therefore, this experimental study proposes developing very small enteric polymer-coated pellets which can be used to formulate liquid pharmaceutical dosage forms adapted to paediatric patients. OME enteric pellets have been produced using the fluid bed coating technique using three different layers. To determine the optimal conditions of the coating process, the design of experiment (DOE) method was applied, particularly, the full factorial design (FFD) of factors with two levels each and a central point. Our aim was to study the effects of several factors on one or more responses and to find a mathematical model relating the response to the factors [13,15]. As the quality of pharmaceutical products is a prerequisite in any manufacturing process, this study followed the criteria of the quality by design (QbD) method described in the guidelines of the International Conference on Harmonisation (ICH Q8 R2) [16].
Download the full article as PDF here Optimisation of the Manufacturing Process of Organic-Solvent-Free Omeprazole Enteric Pellets for the Paediatric Population: Full Factorial Design
or read it here
Materials
Micronized omeprazole (CAS no. 73590-58-6) was received from Esteve Química, Barcelona, Spain. Lactose monohydrate (CAS no. 10039-26-6), sodium lauryl sulphate (CAS no. 151-21-3), titanium dioxide (CAS no. 13463-67-7), and Talc (CAS no. 14807-96-6) were purchased from Fagron Ibérica SAU, Terrassa, Spain. Vivapur® MCC spheres were purchased from JRS Pharma GmbH & Co. KG, Rosenberg, Germany. Hydroxypropyl methyl cellulose (CAS no. 9004-65-3) and hydroxypropyl cellulose (CAS no. 9004-64-2) were purchased from Shin-Etsu Chemical Co., Ltd., Tokyo, Japan. Eudragit® L-30 D-55 was purchased from Evonik Corp., Barcelona, Spain. Triethyl citrate (CAS no. 77-93-0), disodium dihydrogen phosphate (CAS no. 7558-79-4), sodium dihydrogen phosphate (CAS no. 7558-80-7), sodium tetraborate decahydrate (CAS no. 1303-96-4), tribasic sodium phosphate dodecahydrate (CAS no. 10101-89-0), sodium hydroxide (CAS no. 1310-73-2), disodium hydrogen phosphate 12-hydrate (CAS no. 10039-32-4), hydrochloric acid 5 M (CAS no. 7647-01-0), and ethanol 96% (CAS no. 64-17-5) were purchased from PanReac Química S.L.U., Barcelona, Spain.
Rouaz-El-Hajoui, K.; García-Montoya, E.; López-Urbano, A.; Romero-Obon, M.; Chiclana-Rodríguez, B.; Fraschi-Nieto, A.; Nardi-Ricart, A.; Suñé-Pou, M.; Suñé-Negre, J.M.; Pérez-Lozano, P. Optimisation of the Manufacturing Process of Organic-Solvent-Free Omeprazole Enteric Pellets for the Paediatric Population: Full Factorial Design. Pharmaceutics 2023, 15, 2587. https://doi.org/10.3390/pharmaceutics15112587
Read more on Talc as a pharmaceutical excipient here:


