Palatability and Stability Studies to Optimize a Carvedilol Oral Liquid Formulation for Pediatric Use
Abstract
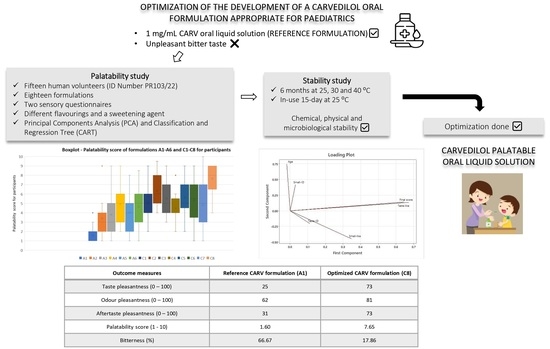
Carvedilol (CARV) is a blocker of α- and β- adrenergic receptors, used as an “off-label” treatment for cardiovascular diseases in pediatrics. Currently, there is no marketed pediatric-appropriate CARV liquid formulation, so its development is necessary. Palatability (appreciation of smell, taste, and aftertaste) is a key aspect to be considered during the development of pediatric formulations since only formulations with good palatability also have adequate acceptability in this population. Consequently, the aim of this research was to assess the palatability and acceptability of different CARV formulations using an in vivo taste assessment (ID Number PR103/22) in order to select the highest palatability-rated CARV formulation.
The preparation of CARV formulations was based on a reference 1 mg/mL CARV solution, which contains malic acid as a solubilizing agent. Subsequently, sucralose and flavoring agents were added and mixed until complete dissolution to the corresponding formulations. Adult volunteers participated in this study and evaluated the taste and odor of various CARV formulations through a questionnaire and a sensory test. The mean palatability score, measured on a 10-point scale, increased from 1.60 for the unflavored control to 7.65 for the highest-rated flavored formulation.
Moreover, the bitterness of the optimized CARV formulation was reduced from 66.67% to 17.86%, and the taste pleasantness was increased from 25/100 to 73/100. This optimized CARV formulation contains a sweetening agent, sucralose, in addition to two flavoring agents at appropriate concentrations for pediatrics. Furthermore, the physicochemical and microbiological stability of the optimized CARV formulation were evaluated for 6 months at 25, 30, and 40 °C, in addition to in-use stability for 15 days at 25 °C, whose results were confirmed. Thus, we successfully developed a palatable CARV liquid solution that contains excipients appropriate for pediatrics and is stable under the studied conditions.
Introduction
Oral liquid formulations are often deemed optimal for pediatric application, a viewpoint also supported by the European Medicines Agency (EMA). This preference primarily stems from their flexibility in dosing and adaptability, allowing drug dosage to be tailored according to the age and weight of each pediatric patient [1]. Designing a pediatric oral formulation is challenging with respect to several issues, such as drug solubility and the selection of excipients, which greatly influence palatability. The palatability of a pharmaceutical formulation refers to its level of pleasantness or acceptability concerning taste, smell, and the overall sensory experience upon administration to patients. Oral pharmaceutical formulations are often unpalatable. Hence, it is often cited as a major cause of non-adherence, especially for geriatric and pediatric populations, who may have swallowing difficulties. An unpalatable formulation can heavily reduce the young patient’s compliance and, consequently, the effectiveness of the therapy [2].
Various taste-masking strategies can be employed for this purpose, and it is important to account for children’s preferences. However, it is crucial to note that certain excipients might elicit adverse reactions in children, particularly when treating infants and neonates. The EMA [1,3] has issued guideline/reflection papers for the pediatric population, while the FDA (Food and Drug Administration) has created a set of guidance documents centered around pediatric patient-focused drug development. Additionally, taste-masking assessments were listed in the contents of the investigation of pediatric drug product development plan measures specified in the pediatric regulations [4,5].
Numerous taste-masking methods exist, yet none universally meet the needs of all drug types, posing a challenge in selecting suitable technology for various Active Pharmaceutical Ingredients (APIs). Taste masking approaches have been primarily categorized based on the mechanism of taste transmission. One approach involves blocking taste transmission pathways, employing methods such as flavoring agents and bitter inhibitors. Generally, the first choice is to use flavoring agents to mask the taste. After that, if the taste is still unacceptable, we should research other methods or a combination of them. The incorporation of flavors serves to mitigate the bitter taste of medicine by competing with the drugs to stimulate taste receptor cells. For example, sweeteners are found to be more effective in masking bitterness compared to acid agents [6,7]. Flavors are categorized as a type of excipient that inherently possesses a pleasant taste and odor. These flavoring agents are commonly classified into sweeteners, acidic flavors, and aromatic agents based on their taste. From the pharmaceutical industry’s point of view, adding flavors is a very attractive method because of its inherently enjoyable taste. There are numerous sweeteners in practical application, such as sucrose, lactose, aspartame, sucralose, mannitol, and saccharin sodium. Meanwhile, the acceptable daily intake (ADI) of sweeteners is also worthy of attention, especially for children. Other frequently used taste-masking techniques for oral formulations include lipophilic vehicles, cyclodextrin complexation, ion exchange resin, liposomes, microcapsules, and nanoemulsions [7,8].
CARV is a non-selective blocker of α- and β-adrenergic receptors used as an ‘off-label’ treatment for clinically treating cardiovascular diseases such as hypertension and congestive heart failure in the pediatric population. Additionally, EMA has included carvedilol on its list of pediatric and therapeutic requirements for cardiology. However, CARV has only been commercialized in an oral solid dosage form as a tablet; hence, the development of a pediatric-appropriate pharmaceutical formulation for CARV is necessary [9]. We successfully developed a stable aqueous CARV liquid formulation suitable for pediatrics [10]. However, optimization of this formulation is the next step, which includes taste assessment and a stability study in order to make CARV formulation more palatable. CARV dissolved in water exhibits an unpleasant bitter taste, which is challenging to mask, making the formulations less palatable. Therefore, palatability was selected as the criterion of choice for a new palatable age-adapted formulation.
CARV tablets are often manipulated prior to use in hospitals with the aim of improving patient compliance and adherence to prepare a CARV liquid suspension [10]. The most common excipient used to prepare a suspension with triturated CARV tablets is a mixture of Ora-Sweet–Ora-Plus (1:1) or Ora-Blend. Some components of this mixture, such as sorbitol, sucrose, and saccharin, are not recommended in pediatrics [11,12]. Ora-sweet gave a sweet citrus-berry flavor to the formulation. Another example is SyrSpend SF PH4 (Fagron), a ready-to-use, all-in-one suspending and sweetening oral liquid vehicle containing cherry flavoring [13].
Operto et al. [14] developed two CARV liquid formulations for administration to pediatric. However, to improve the palatability of these formulations, a small volume of the formulation may be diluted in milk prior to administration. Combining a formulation with food or drinks, such as milk, to mask its unpleasant taste may be considered if it is proven that further improvement is not achievable. However, this can influence the pharmacokinetic behavior of the API [3].
Furthermore, the development of CARV mini tablets could avoid the bitter, unpleasant taste of the API. Khan et al. [15] developed a CARV orally disintegrating mini tablet (ODMT) appropriate for pediatrics, whose doses were 0.5 mg and 2 mg, compared to 3.125 mg as the lowest strength of CARV-marketed tablets. However, oral solutions remain the preferred choice for pediatrics, especially in neonates or children up to 4 years old, as well as for elderly patients who have swallowing difficulties [3].
The taste of a pharmaceutical oral liquid formulation can be quantitatively evaluated using a taste sensor as an electronic tongue (e-tongue) or qualitatively by taste panels. The e-tongue functions were akin to human gustatory sensation, capable of detecting tastes by changes in the electric charge density on the sensor’s membrane surface when exposed to taste substances. The electronic tongue provides an output indicating the taste quality and intensity of the tested formulations compared to predetermined references. Some researchers used an e-tongue for the development of a palatable pediatric formulation [1,16,17].
However, human testing using a sensory questionnaire is acknowledged as the best method to assess the taste of a pharmaceutical formulation. Sensory results of taste assessment in human volunteers may be closer to the real, compared to sensory results using an e-tongue [7]. It is evident that children as a target population are regarded as the most suitable panel for taste assessment of pediatric formulations because children experience different taste sensations than adults [18]. Nevertheless, authorities recommend conducting palatability assessments involving children whenever feasible. When such assessments with children are not possible, taste screening by adult panels can serve as an alternative method, with the results examined for their applicability to children [19]. At this stage of development, we deemed an initial screening by an adult testing panel to be acceptable. Regarding the palatability evaluation by healthy volunteers, each healthy adult volunteer was asked to evaluate their taste and odor perception using a questionnaire rating scale.
Considering these findings, the goal of this work was the development of an oral palatable solution of CARV for pediatric use, exploiting taste masking in association with different flavoring agents and a sweetener. Different combinations of flavorings and a sweetening agent were selected to prepare CARV solutions and were subject to a human taste assessment to evaluate their actual ability to mask the unpleasant taste of the CARV in comparison with the reference CARV solution [10].
The aim of this research was to select the CARV solution with the highest palatability score and least bitterness level for healthy human volunteers. The highest palatability-rated CARV formulation for the participants was included in a stability study for 6 months and evaluated in terms of chemical, physical, and microbiological stability under 25, 30, and 40 °C. Additionally, a 15-day in-use stability study of this formulation was conducted at 25 °C.
Download the full article as PDF here: Palatability and Stability Studies to Optimize a Carvedilol Oral Liquid Formulation for Pediatric Use
or read it here
Chiclana-Rodríguez, B.; Garcia-Montoya, E.; Romero-Obon, M.; Rouaz-El-Hajoui, K.; Nardi-Ricart, A.; Suñé-Pou, M.; Suñé-Negre, J.M.; Pérez-Lozano, P. Palatability and Stability Studies to Optimize a Carvedilol Oral Liquid Formulation for Pediatric Use. Pharmaceutics 2024, 16, 30. https://doi.org/10.3390/pharmaceutics16010030
Read more articles on the formulation of Carvedilol here:
- 3D printing of carvedilol oral dosage forms using selective laser sintering technique
- Development of a Carvedilol Oral Liquid Formulation for Paediatric Use
- 3D Printing of Personalised Carvedilol Tablets Using Selective Laser Sintering