3D Printing of Personalised Carvedilol Tablets Using Selective Laser Sintering
Abstract
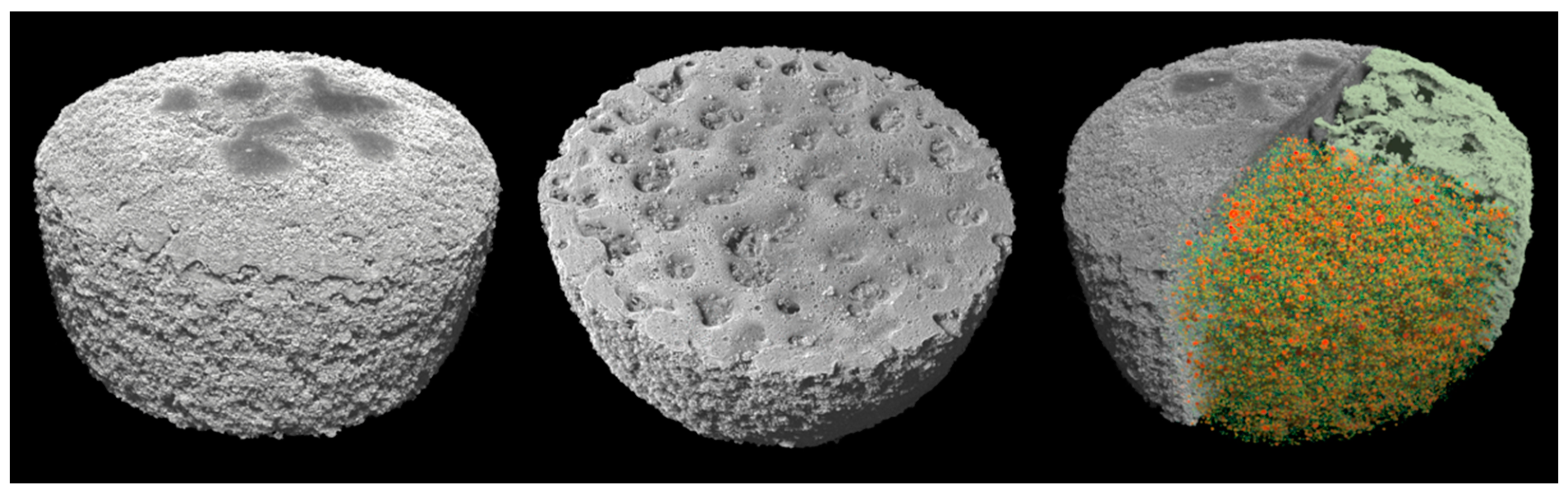
Selective laser sintering (SLS) has drawn attention for the fabrication of three-dimensional oral dosage forms due to the plurality of drug formulations that can be processed. The aim of this work was to employ SLS with a CO2 laser for the manufacturing of carvedilol personalised dosage forms of various strengths. Carvedilol (CVD) and vinylpyrrolidone-vinyl acetate copolymer (Kollidon VA64) blends of various ratios were sintered to produce CVD tablets of 3.125, 6.25, and 12.5 mg. The tuning of the SLS processing laser intensity parameter improved printability and impacted the tablet hardness, friability, CVD dissolution rate, and the total amount of drug released. Physicochemical characterization showed the presence of CVD in the amorphous state. X-ray micro-CT analysis demonstrated that the applied CO2 intensity affected the total tablet porosity, which was reduced with increased laser intensity. The study demonstrated that SLS is a suitable technology for the development of personalised medicines that meet the required specifications and patient needs.
Introduction
Three-dimensional printing has transformed manufacturing capabilities due to its widespread applications in the automotive [1], biomedical, aerospace [2], and pharmaceutical [3] industries, as well as in art [4]. Over the past decade, there has been a growing interest in the use of 3D printing technologies for pharmaceutical applications, and particularly for the design and fabrication of personalised medicines at the point of care. A wide range of 3D printing techniques such as fused deposition modelling (FDM) [5,6,7], material jetting (MJ) [8], selective laser sintering/melting (SLS/SLM) [9], stereolithography (SLA) [10], micro extrusion [11,12], and/or combinations thereof [13] have been employed in pharmaceutical research.
Despite the small scale capabilities, 3D printing presents several advantages over conventional tableting, including the fabrication of dosages with tailored drug amounts, sizes, shapes, and complex geometries, with specific release profiles [14,15]. Hence, 3D-printed dosage forms can be used for designing individualised patient treatments that meet clinical needs, improve patient compliance, and improve the treatment of diseases [16]. The versatility of 3D printing technologies is evidenced by the numerous studies such as the development of paediatric chewable formulations [12,17], bilayer tablets [18], polypills [19,20], or even orally disintegrating tablets [21].
FDM is the most frequently used printing technology for the development of complex tablet designs or compartmental dosage forms with tuneable drug dissolution rates. Nevertheless, FDM largely depends on the extrudability of pharma grade thermoplastic polymers, APIs, and the printability of the formed API loaded filaments, and drug loading can also be a limiting factor [22,23,24]. Regardless of the numerous 3D printing applications in the design of oral solid dosage forms, the use of SLS/SLM technologies for pharmaceutical applications has not been fully exploited. In SLS/SLM technologies, the printing chamber and the powder reservoir of an SLS printer are both heated to a temperature below the melting point (Tm) or the glass transition (Tg) of the printable powder. The top layer of powder in the preheated printing chamber is then exposed to a high-power X-Y axis laser beam, which starts to sinter a predetermined 2D pattern in accordance with the product design. The process is repeated, and another thin layer of powder is dispersed once each layer has been successfully completed. The printing is attained by lowering the printing chamber to a predetermined height and rising the reservoir chamber to a predetermined height. The recoating roller/blade can then apply a fresh powder surface on top of the completed layer. A major advantage of SLS is the reduced number of processing steps (e.g., milling) and excipients in comparison to other printing technologies. Those features render SLS simpler, inexpensive, and flexible, with reduced processing times and material losses. Most importantly, its operational simplicity and small footprint renders SLS an appealing printing technology for point-of-care applications [12]. Fina et al. used SLS to investigate the printing of Kollicoat IR and Eudragit L100-55 paracetamol formulations at 5%, 20%, and 35% loadings by weight. The blends were processed at various intensities to avoid drug degradation, and 3% by weight Candurin® gold sheen was added [25]. Depending on the laser intensity, the produced paracetamol tablets can feature various release profiles due to the different tablet porosities that can be obtained for the two polymers. In another study, the printability of paracetamol tablets with polyethylene oxide, Eudragit (L100-55 and RL), and ethyl cellulose was systematically investigated.
The experimental findings revealed that the paracetamol release varied based on the polymer grade, laser scanning speed, and the tablet’s designed shape, such as solid cylindrical, gyroid, and bilayer structures [26]. However, the major disadvantage of these studies was the inadequate paracetamol amounts in the printed printlets and the slow dissolution rates that did not comply with pharmacopeia standards.
Recently Gueche et al. [27] investigated the printing of paracetamol with Kollidon VA64/polyamide 12 blends for the formation of fast-disintegrating dosage forms using an SLS printer equipped with a CO2 laser printer. The use of different paracetamol grades with large and plate-like particles or thin and needle-like particles affected the porosity and the dissolution rates of the sintered tablets. In addition, the drug–polymer ratio, the drug loading, and the particle size were found to be key critical material parameters.
To our knowledge, there are no reported studies using SLS for the development of personalised dosage forms. Therefore, the aim of this work was to investigate the print-on-demand capabilities of SLS technology for developing personalised dosage forms that meet pharmacopeia specifications in terms of strength, friability, active dose, and dissolution rate. Carvedilol is used to treat heart failure and hypertension, and it was selected as the model drug substance. The printing process parameters were optimised for loadings of 3.125, 6.25, and 12.5 mg respectively.
Download the full article as PDF here 3D Printing of Personalised Carvedilol Tablets Using Selective Laser Sintering
or read it here
Materials
Kollidon VA 64 (VA64) was kindly donated by BASF (BASF-Germany) and carvedilol (CVD) was purchased from Tokyo Chemicals (Japan).
Tabriz, A.G.; Gonot-Munck, Q.; Baudoux, A.; Garg, V.; Farnish, R.; Katsamenis, O.L.; Hui, H.-W.; Boersen, N.; Roberts, S.; Jones, J.; et al. 3D Printing of Personalised Carvedilol Tablets Using Selective Laser Sintering. Pharmaceutics 2023, 15, 2230. https://doi.org/10.3390/pharmaceutics15092230
Read more on Overview of Pharmaceutical 3D printing here:


