Long-acting Parenteral Drug Delivery Systems for the Treatment of Chronic Diseases
Abstract
The management of chronic conditions often requires patients to take daily medication for an extended duration. However, the need for daily dosing can lead to nonadherence to the therapy, which can result in the recurrence of the disease. Long-acting parenteral drug delivery systems have the potential to improve the treatment of chronic conditions. These systems use various technologies, such as oil-based injectables, PLGA-based microspheres, and in situ forming gel-based depots, to deliver different types of drugs. The use of long-acting parenteral formulations for the treatment of chronic infections such as HIV/AIDS and tuberculosis is a recent development in the field. Researchers are also exploring the use of long-acting parenteral formulations for the treatment of malaria, with the aim of reducing dosing frequency and improving adherence to treatment. This review discusses various aspects of long-acting formulation development, including the impact of the physicochemical properties of the drug, the type of long-acting formulation, and the route of administration. The clinical significance of long-acting formulations and recent advances in the field, such as long-acting nanoformulations and long-acting products currently in clinical trials, have also been highlighted.
Introduction
Management of several chronic diseases, including schizophrenia, diabetes, and HIV/AIDS, requires the administration of drugs on a daily basis for the entire life of a patient. The majority of the drugs used for the management of these diseases are available as oral formulations. The oral route usually offers the highest level of compliance among all other routes of administration. Yet a large number of patients often find it difficult to adhere to a daily oral dosage regimen for a prolonged period. The issue of non-adherence becomes even worse when the daily dosing frequency is more than once. However, once-a-day oral formulations are able to tackle the issue to some extent. It is still considered high when the treatment duration is significantly prolonged. Interestingly, Kirtane et. al. reported an oral long-acting formulation for the delivery of six antiretroviral drugs simultaneously for a week. The dosage form consisted of six arms consisting of drug-polymer matrices attached to a central core, which could achieve a week-long systemic drug concentration in vivo in a pig model [1]. However, the technology was far from clinical translation.
In addition to the high dosing frequency, the ineffectiveness of the drug after oral administration is another challenge in oral therapy. For example, therapeutic proteins and peptides including liraglutide, exenatide, and octreotide exhibit extremely poor oral bioavailability due to instability in the gastrointestinal tract and hence are administered subcutaneously once or twice a day [2], [3], [4]. In this case, patient compliance is further reduced due to the invasive nature of the route. Researchers are making continuous efforts toward the successful oral delivery of therapeutic proteins and peptides [5]. It is interesting to note that recently Novo Nordisk succeeded to get the US Food and Drug Administration as well as the European Medicines Agency (EMA) approval for semaglutide oral tablets for the treatment of type 2 diabetes mellitus. However, it showed a very low oral bioavailability (0.4 – 1%) of semaglutide [6], [7]. This poor bioavailability of semaglutide may be the due to combination of various factors including digestion of peptide by proteolytic enzymes present in the gastrointestinal tract as well as poor permeability due to high molecular weight (4113.58 g/mol) and poor lipophilicity. If a patient wanted to shift from a once-a-day semaglutide oral tablet to a subcutaneous injection, a 0.5 mg/week dose of semaglutide would be sufficient [8]. Similarly, in the case of octreotide, similar systemic exposure was observed between a single oral dose of 20 mg and a single subcutaneous injection of 0.1 mg [9]. Hence, it is clearly evident that even an invasive route, e.g., subcutaneous/intramuscular, can be an affordable solution for an expensive drug. In addition, an invasive route can also provide enhanced patient compliance in the case of semaglutide by reducing dosing frequency. However, it is to be noted that an extended duration of semaglutide is based upon covalent structural modification in GLP-1 and such modifications may not be feasible or favourable in terms of safety/efficacy of other drugs along with the limitation of very high development cost. For example, in the case of octreotide, the initial dosage is usually 50 mcg administered as a subcutaneous injection either twice or thrice a day [4]. Considering patient compliance, it would certainly be very challenging.
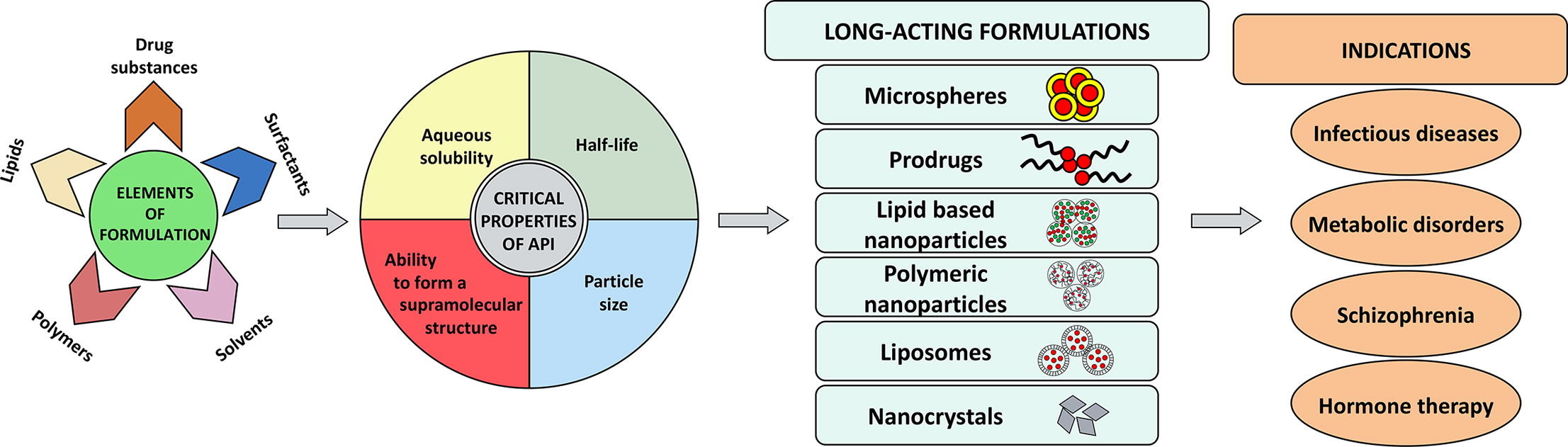
Long-acting injectable (LAI) formulations can serve as a platform technology for a wide range of drugs, offering the additional benefit of cost-effectiveness. This review discusses LAI formulations administered parenterally via intramuscular and subcutaneous routes to achieve sustained plasma drug concentrations ranging from a few weeks to several months. However, this review does not cover LAI formulations administered by other routes, such as transdermal, nasal, ocular and pulmonary, as they fall outside the scope of this review. LAI formulations provide unique advantages, including extended drug exposure, minimal fluctuations in plasma drug concentration, and reduced frequency of administration. Over the past few decades, various technologies have been developed to accommodate different types of drugs in LAI formulations. For example, oil-based LAI formulations were developed in the 1960s to 1980s but were limited to the delivery of hydrophobic/lipophilic drugs. To deliver hydrophilic drugs, specifically therapeutic proteins and peptides, PLGA-based microsphere technology was developed in the 1990s. However, microspheres have inherent limitations, and the technology was patent protected, leading to the development of in situ forming gel-based depot technology from 1998 to 2005 as an alternative. Since 2005, small, less hydrophobic drugs have been converted into highly hydrophobic prodrugs and formulated as simple aqueous nano/micro suspension-based LAI formulations [10]. Table 1 lists the different LAI formulations that have been available on the market since 2013. Additionally, supplementary Table S1 includes information on LAI formulations that were approved prior to 2013. Among the available LAI formulations for clinical use since 2013, aqueous suspension or polymeric microspheres account for the majority of developed technologies. However, compared to the products approved before 2000, the number of oil-based LAI formulations has decreased. In recent years, several new technologies have emerged to overcome the limitations associated with already-approved products. One such example is SUSTOL. When developing LAI formulations, it is important to consider the injection volume. For LAI products approved since 2013, a maximum injection volume of 5.0 mL for the intramuscular route and 1.5 mL for the subcutaneous route can be administered. Recently, two aqueous suspension-based LAI formulations have been approved for the treatment of HIV infection. These are the only LAI products approved for treating chronic infections, which validate the application of LAI formulations for treatment of chronic infections.
The development of LAI formulations is a complex, time-consuming, and costly process. One of the challenges in the development of LAI formulations is the limited selection of polymers and excipients that are available. As a result, some innovator companies develop proprietary excipients for use in LAI formulations, which can delay the development of generic LAI products. For example, SANDOSTATIN® (Octreotide acetate) LAR DEPOT, a microsphere-based LAI formulation, uses a proprietary biodegradable polymer that has not yet been used in any generic LAI formulation, even 24 years after its introduction [4]. Similarly, SUSTOL (Granisetron) is the first high-viscosity-based LAI formulation, which was approved by the US Food and Drug Administration in 2016 and uses proprietary polymers and excipients for sustained drug release [41]. Overall, the complex nature of LAI formulation development, including the use of proprietary excipients, can significantly impact the availability of generic LAI products.
While few research groups have published reviews on the development of LAI formulations and their clinical significance, some aspects still have not received sufficient attention. For example, the physicochemical and pharmacological properties of drugs on the development of LAI formulations currently available for various clinical conditions have not been fully elaborated. A more comprehensive understanding of these properties is necessary to optimize the design and efficacy of LAI formulations. Additionally, LAI formulations have shown great potential in the management of chronic infections, yet this potential has not been adequately discussed in the literature [43]. This review provides a comprehensive description of the key considerations for the development of long-acting parenteral drug delivery systems. It covers a range of important factors, such as the physicochemical properties of the drug substance, the route of administration (intramuscular vs. subcutaneous), and the type of long-acting formulation used. Moreover, the review specifically focuses on the advancements made in long-acting nanocarrier-based drug delivery systems, highlighting the recent developments in this field. This section provides valuable insights into the various strategies and technologies used to achieve sustained drug release and improved therapeutic outcomes. In addition, the review elaborates on the clinical significance of long-acting drug delivery systems, including their potential benefits for patients and healthcare providers.
Read more here
Excipients mentioned in the study beside others: Tween 80, Mannitol, Carmellose Sodium
Anil B. Jindal, Atharva R. Bhide, Sagar Salave, Dhwani Rana, Derajram Benival, Long-acting Parenteral Drug Delivery Systems for the Treatment of Chronic Diseases, Advanced Drug Delivery Reviews, 2023, 114862, ISSN 0169-409X, https://doi.org/10.1016/j.addr.2023.114862.
Read more on Excipients for Parenterals here:


