Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID‐19 vaccine
On the first day of the UK vaccination campaign with the coronavirus disease 2019 (COVID‐19) vaccine, there were reports of 2 cases of anaphylaxis within minutes of administration of the Pfizer/BioNTech messenger RNA (mRNA) vaccine and a third case of an allergic reaction not requiring adrenaline (epinephrine). This was alarming, as anaphylaxis to vaccines is rare, in the order of 1 case per million doses,1 and therefore likely to injure public confidence. The UK Medicines and Healthcare products Regulatory Agency (MHRA) issued precautionary advice restricting access to the vaccine,2 which was subsequently relaxed in line with the summary of product characteristics. The cause of these vaccine anaphylaxis cases is unclear, but polyethylene glycol (PEG) is a candidate allergen.3, 4 Here, we demonstrate for the first time that allergy to PEG can cause anaphylaxis to the Pfizer/BioNTech vaccine.
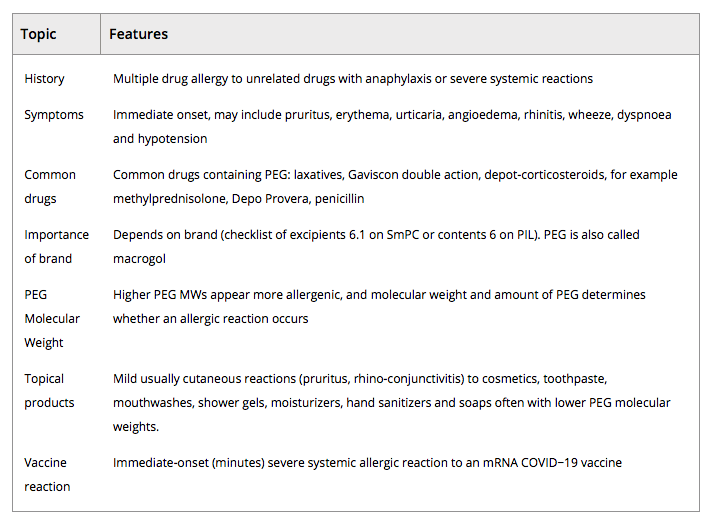
Allergy to PEG is rare,5, 6 but reactions can be severe or even fatal.7 PEG is an excipient of many drugs, but hypersensitivity depends on its molecular weight (MW).
A 52‐year‐old woman developed throat constriction, cough and then loss of consciousness immediately after receiving the Pfizer/BioNTech COVID‐19 vaccine. She had a respiratory rate of 30/min, tachycardia of 150/min and oxygen saturation of 85%. Her blood pressure became unrecordable although central pulse remained palpable. She was treated with 2 doses of intramuscular adrenaline 0.5 mg, intravenous hydrocortisone 200 mg, chlorphenamine 10 mg, and fluids and oxygen 15 litres/min. Tryptase taken 1.5 hours later was 3.9 ng/ml (normal range 2–14 ng/ml).
She gave a history of allergic reactions to multiple products including drug anaphylaxis. Three years ago, after a first dose of azithromycin containing PEG 6000, she developed facial swelling, widespread urticaria and wheezing. Adrenaline was administered, and she was taken to hospital. She described urticaria with shampoos, conditioners, shower gels containing PEG and immediate burning of the mouth with toothpastes and mouthwash containing PEG. She had never received products such as laxatives, depo‐progesterone or depo‐methylprednisolone containing PEG 3500. She was investigated in our drug allergy clinic 15 days after vaccination. She was not known to be PEG allergic prior to vaccination, but due to the severity of her reaction and previous history we suspected PEG allergy.
Skin prick testing was undertaken to PEG 400, 600, 2000, 3350, 4000, 6000, 8000 and 20,000 at 0.1% and 1% (w/v in Coca’s solution) and polysorbate 80 at 10% and 20% (Thermo Fisher Scientific). We also tested the Pfizer/BioNTech vaccine, excipients of the vaccine (1,2‐distearoyl‐sn‐glycero‐3‐phosphocholine and cholesterol; Sigma‐Aldrich Co), and the AstraZeneca COVID‐19 vaccine. Vaccine testing used the residual from freshly made‐up vials, and therefore, no vaccine dose was wasted.
Skin prick tests were negative to all PEGs at 0.1% concentration and to the Pfizer/BioNTech vaccine, its other excipients, polysorbate 80 and the AstraZeneca COVID‐19 vaccine. Testing was positive to PEG 4000 at 1% of concentration (7‐mm wheal and 25‐mm flare). Twelve minutes after skin testing, and 2 minutes after the skin test became positive, she developed a systemic reaction with widespread pruritus, urticaria and coughing with throat constriction. Systolic blood pressure dropped from 137 mm Hg to 107 mm Hg. She was treated in clinic with intramuscular adrenaline 0.5 mg, intravenous chlorphenamine 10 mg and hydrocortisone 200 mg. Her blood pressure improved but coughing persisted, with a drop in oxygen saturation to 85%. A second dose of intramuscular adrenaline 0.5 mg was administered, and she rapidly improved. Tryptase levels measured immediately, 1 hour and 3 hours after this episode were 5.5, 5.5 and 4 ng/ml, respectively. PEG allergy was diagnosed as the cause of her Pfizer/BioNTech vaccine anaphylaxis.
Although the Pfizer/BioNTech vaccine contains a number of excipients, PEG 2000 is the only one reported to cause anaphylaxis. All mRNA vaccines are likely to contain PEG, which is used to stabilize the lipid nanoparticles, and the Moderna COVID‐19 vaccine also contains PEG 2000.8 These are the first mRNA vaccines to be licensed in UK, so there is no prior information on allergic reactions to mRNA vaccines. Other mRNA COVID‐19 vaccines are in development.9 As far as we can determine, PEG has not been used as an excipient in vaccines until now. Polysorbate 80 is an excipient in the Oxford/AstraZeneca vaccine. There has been a suggestion of cross‐reactivity between PEG and polysorbate 80 due to their similar chemical structures. For example, Garvey found 3 of 10 PEG‐allergic patients had positive skin prick tests to polysorbate 80 at 20%.10 It is unclear whether this translates into clinical cross‐hypersensitivity. Polysorbate 80 is used as an excipient in many drugs (including some vaccines such as influenza) and as a food additive and is thus widely tolerated.
To our knowledge, this is the first demonstration that anaphylaxis to the Pfizer/BioNTech vaccine can be due to PEG allergy. The case had a large positive skin prick test to PEG 4000 and systemic reaction on skin prick testing, confirming a reproducible response to PEG. Mast cell tryptase level did not increase in the index reaction or in the skin test–induced anaphylaxis. This might be because the severe features of the skin test reaction were predominantly respiratory as in food anaphylaxis where tryptase is often within the normal range, but does not explain the vaccine reaction.11 Diagnosis of PEG allergy is challenging, with most PEG‐allergic patients presenting with severe anaphylaxis to multiple unrelated drugs.6 Our patient’s history of multiple drug allergic reactions with sudden‐onset anaphylaxis supported a diagnosis of PEG allergy. She also reacted to PEG in shower gels, shampoos, toothpaste and mouthwash.
Continue reading here: Sellaturay, P., Nasser, S., Islam, S., Gurugama, P. and Ewan, P.W. (2021), Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID‐19 vaccine. Clin Exp Allergy. https://doi.org/10.1111/cea.13874
TABLE 1. The clinical features of a PEG‐allergic patient may help identify at‐risk patients before vaccination or if a COVID‐19 vaccine reaction was likely due to PEG