Development and Characterization of Pentaerythritol-EudragitRS100 Co-processed Excipients as Solid Dispersion Carriers for Enhanced Aqueous Solubility, In Vitro Dissolution, and Ex Vivo Permeation of Atorvastatin
Abstract
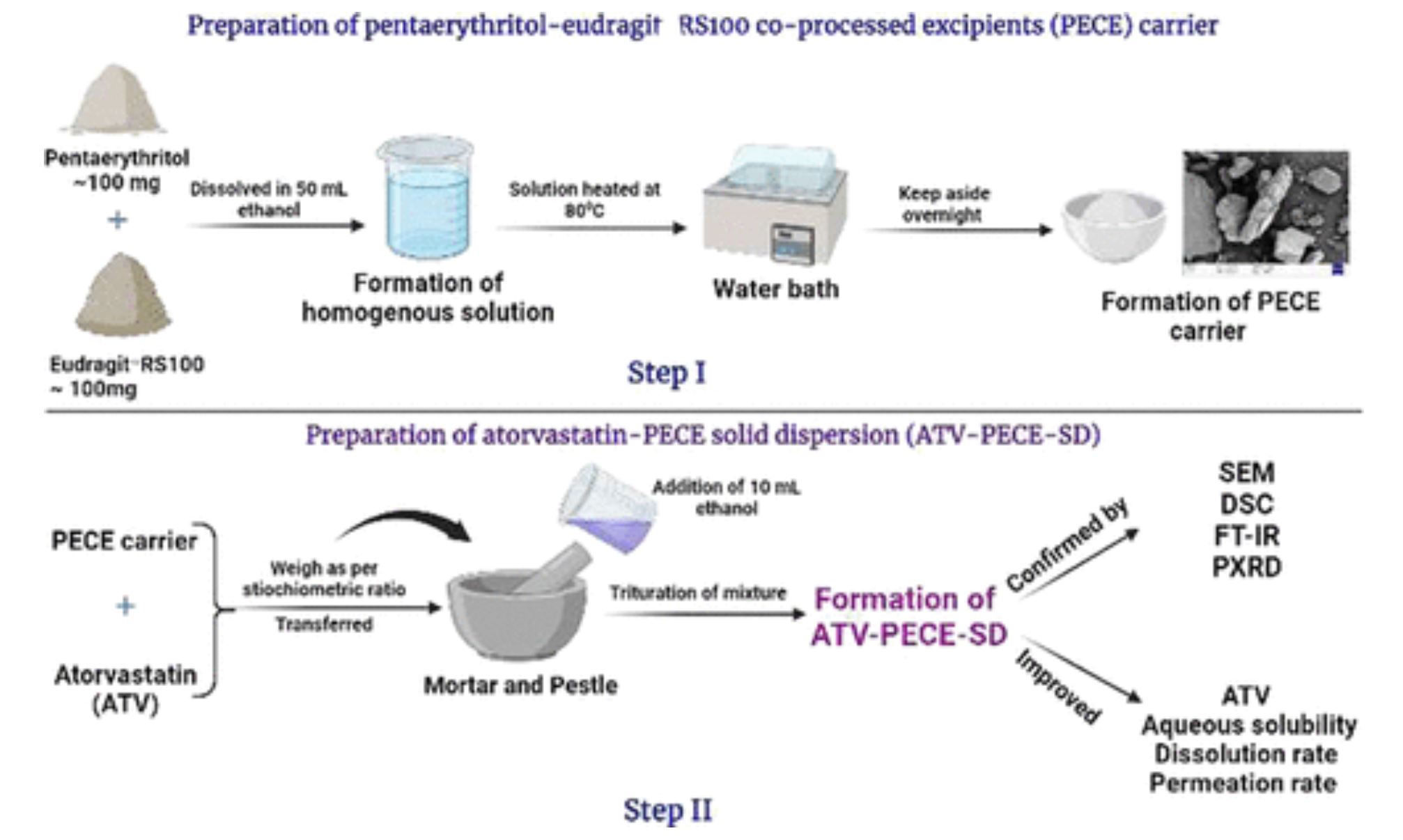
Atorvastatin (ATV), a lipid-lowering agent, has low oral bioavailability due to its poor water solubility, permeability, and low dissolution rate. Therefore, pentaerythritol-EudragitRS100 co-processed excipients (PECE) were synthesized, and their feasibility as solid dispersion carriers (ATV-PECE-SD) for improving the solubility, permeability, and dissolution rate of ATV was explored. Solid dispersions were assessed in terms of particle size and zeta potential, and solubility, in vitro dissolution, and ex vivo permeation studies were studied. Scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FT-IR), differential scanning calorimetry (DSC), and powder X-ray diffraction (PXRD) were used as characterization tools. ATV-PECE-SD3 (1:4) formulations exhibited a small particle size with high stability. Physicochemical evaluation evidenced the formation of solid dispersion due to the involvement of weak electrostatic interaction between the polar functional groups of ATV and PECE carriers. ATV-PECE-SD3 (1:4) significantly enhanced the water solubility by ∼43-fold compared to pure ATV. In vitro dissolution studies showed that optimized formulation enhanced the dissolution rate of ATV compared to pure ATV. Ex vivo permeation results revealed that ATV-PECE-SD3 (1:4) enhanced the permeation rate of ATV compared to pure ATV. The optimized formulations significantly improved the dissolution rate of ATV in the fed state due to the food effect and micelle formation mechanism compared to the fasted state. The study concludes that co-processed excipients could be used as promising solid dispersion carriers to enhance the aqueous solubility, permeability, and dissolution rate of ATV.
Introduction
Atorvastatin (ATV) (IUPAC name: (3R,5R)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenyl carbamoyl)-5-(propan-2-yl)-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoic acid) HMG-CoA reductase enzyme inhibitor reduces the cholesterol production by preventing HMG-CoA conversion to mevalonate. (1) Despite these encouraging health benefits, ATV has a low oral bioavailability of about ∼12%, which could be attributed to its poor water solubility of ∼0.1 mg/mL, extensive first-pass metabolism, crystalline behavior, and short half-life of ∼1–2 h, respectively. (2) Due to its limited water solubility and high intestinal permeability profile, ATV is categorized as a biopharmaceutical class II drug. According to reports, ATV poor water solubility and shorter half-life cause an increase in dose, and serious side effects include arthralgia, rhabdomyolysis, liver problems, and renal failure. (3) Therefore, we synthesized and developed a solid dispersion carrier with the goal to enhance the poor water solubility, permeability, and other biopharmaceutical attributes of ATV.
Many formulation approaches have been reported to enhance the ATV solubility, permeability, and dissolution rate. These include tablets, (4) solid dispersion, (1) nano-solid dispersion, (5) semi-solid binary systems, (6,7) nanocrystals, (8) and binary and ternary solid dispersion. (9) A comprehensive examination of different formulation techniques revealed a small improvement in the biopharmaceutical properties of ATV but with many shortcomings. For instance, neem gum-based solid dispersion of ATV improved its limited aqueous solubility by ∼3-fold and ∼4-fold compared to pure ATV. (1) The electrospinning approach had a significant impact on a nano-solid dispersion of ATV/ezetimibe with PVP K30 at a ratio of (1:1 and 1:5). This study focused on a few physicochemical assessments without looking into ATV aqueous solubility, permeability, and oral bioavailability. (5) The development of ATV semi-solid dispersion utilizing Gelucire 44/14 and 50/13 simply improved the absorption and half-life of ATV without assessing its formulation stability. (6) ATV solid dispersion with polyethylene glycol 4000 and 10,000 has also been reported to impart high viscosity and significant toxicity to the formulations. (10) According to reports, poloxamer-based solid dispersion can cause particle aggregation and structural changes in the formulation under varied temperatures and concentrations. (11) The observed shortcomings and noneffectiveness of the existing formulations guide us to develop the co-processed excipients as solid dispersion carriers with enhanced ATV water solubility, permeability, and dissolution rate.
Co-processed excipients are multifunctional excipients created by combining two or more existing excipients at the subparticle level, resulting in a single composite excipient with greater functionality when compared to a physical mixing of the same excipient combination. (12) It improved flow properties, compressibility, dilution potential, disintegration properties, and reduced lubrication sensitivity. Together with these benefits, co-processed excipients have also been reported to enhance the solubility, wettability, stability, and gelling properties of food ingredients, excipients, and active pharmaceutical ingredients. (13) Due to these advantages, researchers have been more interested in using co-processed excipients as solid dispersion carriers to improve the biopharmaceutical characteristics of drugs that are weakly water soluble. The solid dispersion formulation remarkably improved the solubility, permeability, dissolution, and pharmacokinetic profile of poorly aqueous soluble drugs (14−16) by reduction of drug particle size to submicron particles, changing the crystalline state to high-energy amorphous state, and improving the wettability, solubility, and dissolution rate of the drug. (17) Previous reports have shown the use of co-processed excipients as solid dispersion carriers with an enhanced dissolution rate of glimepiride, a poorly water-soluble drug. (18) Pentaerythritol-EudragitRS100 co-processed excipients (PECE) were investigated in the current study as potential solid dispersion carriers for improved ATV solubility, permeability, and dissolving rate. The PECE carrier, in association with the solvent evaporation method, converts the crystalline ATV particles into high-energy state amorphous powder, which increases the drug particle solubility and dissolution rate due to an increase in the surface area. Pentaerythritol is a crystalline, highly aqueous soluble ∼56 mg/mL compound with molecular formula C5H12O4.
Pentaerythritol offers maximum encapsulation and accommodation to all crystalline molecules due to its structural similarity and low lattice energy. This mechanism of pentaerythritol improved the solubility and dissolution rate of the drug through its dispersion and partial or complete amorphization. (19) EudragitRS100 (ERS100) is a cationic copolymer. The presence of ∼4.5–6.8% of functional quaternary ammonium groups on this polymer creates strong intermolecular interaction with a negative drug charge. This interaction improved the drug encapsulation within the polymer and resulted in the formation of a drug–copolymer complex, which allows for controlled, prolonged, and localized drug delivery. (20) Pentaerythritol and copolymer have the ability to deliver drugs, but their combined and therapeutic uses as solid dispersion carriers have not been fully investigated. Research groups of Chiou et al. and Barzegar-Jalali et al. (21,22) have given a few publications on using pentaerythritol and copolymer as solid dispersion carriers. These studies, however, lacked a thorough and systematic physicochemical and functional characterization of formulations. Therefore, the PECE carrier was prepared, and its feasibility as a solid dispersion carrier was explored.
The carrier was synthesized via chemical interaction (i.e., ion-pair and hydrogen bonding) between 5% quaternary ammonium groups of copolymer and hydroxyl groups of pentaerythritol in the presence of ethanol. This interaction further improved the accommodation of the amorphous copolymer within the crystal lattice of pentaerythritol and resulted in the formation of a PECE carrier with high dominance of pentaerythritol. The PECE carrier, which contains 5% quaternary ammonium and hydroxyl groups of copolymers and pentaerythritol, may produce strong electrostatic interactions with the COOH, N–H, and O–H groups of ATV in solid dispersion formulations. This interaction facilitated the accommodation of highly crystalline ATV within the crystal lattice of pentaerythritol, resulting in complete amorphization and the formation of molecular dispersion with enhanced ATV solubility and dissolution rate.
This is a “proof-of-concept” study that intends to investigate the feasibility of PECE as a solid dispersion carrier to improve the ATV aqueous solubility, permeability, and dissolution rate. This work was carried out in two steps. First, the PECE carrier was synthesized using the solvent evaporation method, and then the PECE-based solid dispersion of ATV was developed using the same methodology. The particle size, Fourier transforms infrared spectrophotometry, scanning electron microscopy, differential scanning calorimetry, solubility analysis, and in vitro dissolution studies were used to evaluate the optimized formulations. Also, the same formulations were evaluated for preliminary stability studies under temperature and relative humidity conditions.
Download the full article as PDF here Development and Characterization of PentaerythritolEudragitRS100 Co-processed Excipients as Solid Dispersion Carriers
or read it here
Materials
ATV of high purity (>99%) was obtained from Alkem Laboratories Ltd. (Mumbai, India). Pentaerythritol was obtained from Sigma-Aldrich Corporation (St. Louis, MO). Eudragit RS100 was obtained from Chemsworth Pvt. Ltd. (Bangalore, India). Other chemicals such as calcium chloride, dichloromethane, ethanol, glacial acetic acid, glucose, methanol, n-octanol, potassium chloride, potassium dihydrogen phosphate, sodium chloride, sodium bicarbonate, sodium hydroxide, soya lecithin, and sodium taurocholate were purchased from Loba Chemicals Pvt. Ltd., (Mumbai, India). The purchased chemicals were of analytical grade.
Development and Characterization of Pentaerythritol-EudragitRS100 Co-processed Excipients as Solid Dispersion Carriers for Enhanced Aqueous Solubility, In Vitro Dissolution, and Ex Vivo Permeation of Atorvastatin, Darshan R. Telange, Neha M. Bhaktani, Atul T. Hemke, Anil M. Pethe, Surendra S. Agrawal, Nilesh R. Rarokar, and Shirish P. Jain, Cite this: ACS Omega 2023, Publication Date:July 5, 2023, https://doi.org/10.1021/acsomega.3c02280
© 2023 The Authors. Published by American Chemical Society
Read more on Sodium Stearyl Fumarate as a pharmaceutical excipient here:


