Phase homogeneity in ternary amorphous solid dispersions and its impact on solubility, dissolution and supersaturation – Influence of processing and hydroxypropyl cellulose grade
Abstract
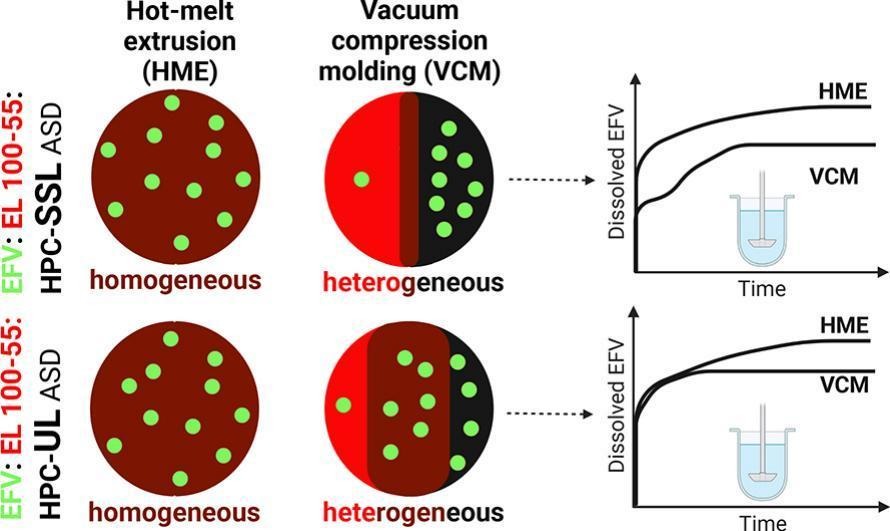
As performance of ternary amorphous solid dispersions (ASDs) depends on the solid-state characteristics and polymer mixing, a comprehensive understanding of synergistic interactions between the polymers in regard of dissolution enhancement of poorly soluble drugs and subsequent supersaturation stabilization is necessary. By choosing hot-melt extrusion (HME) and vacuum compression molding (VCM) as preparation techniques, we manipulated the phase behavior of ternary efavirenz (EFV) ASDs, comprising of either hydroxypropyl cellulose (HPC)-SSL or HPC-UL in combination with Eudragit® L 100–55 (EL 100–55) (50:50 polymer ratio), leading to single-phased (HME) and heterogeneous ASDs (VCM). Due to higher kinetic solid-state solubility of EFV in HPC polymers compared to EL 100–55, we visualized higher drug distribution into HPC-rich phases of the phase-separated ternary VCM ASDs via confocal Raman microscopy. Additionally, we observed differences in the extent of phase-separation in dependence on the selected HPC grade. As HPC-UL exhibited decisive lower melt viscosity than HPC-SSL, formation of partially miscible phases between HPC-UL and EL 100–55 was facilitated. Consequently, as homogeneously mixed polymer phases were required for optimal extent of solubility improvement, the manufacturing-dependent differences in dissolution performances were smaller using HPC-UL, instead of HPC-SSL, i.e. using HPC-UL was less demanding on shear stress provided by the process.
Highlights
- Only hot-melt extrusion led to homogeneous ternary ASDs.
- Preferred drug distribution into HPC-rich phases of heterogeneous VCM ASDs.
- Lower melt viscosity of HPC-UL compared to -SSL facilitated VCM polymer miscibility.
- Miscible polymer phases were decisive for optimal dissolution.
- Lower manufacturing-dependent differences in dissolutions using the HPC grade -UL.
Introduction
The preparation of amorphous solid dispersions (ASDs) is one of the most common and popular formulation principles to overcome bioavailability limitations due to low solubility by generating and maintaining a supersaturated state of the drug in the dissolution (Bhujbal et al., 2021; Mishra et al., 2015; Pandi et al., 2020). During the ASD preparation process the poorly soluble drug is incorporated amorphously into a polymer matrix, mostly consisting of a single polymer (binary ASDs) (Chiou and Riegelman, 1971; Meng et al., 2015). To enable a complete amorphous stabilization and to prevent recrystallization/ phase-separation, the individual solid-state solubility of the drug of interest within the selected polymer must be considered. Immiscibility or the exceedance of the solubility of the drug would lead to the formation of amorphous or crystalline drug-rich phases (Alzahrani et al., 2022; Li et al., 2016; Sharma et al., 2021), and to a pronounced reduction of the supersaturation performance (Wlodarski et al., 2018). Regarding the solubility enhancement, an optimal dissolution of an ASD is described by a high initial dissolution rate, followed by an inhibition of precipitation and stabilization of the supersaturated state (Brouwers et al., 2009; Hanada et al., 2021). As similar to the solid-state stabilization generating and maintaining supersaturated drug solutions upon dissolution dependent on specific interactions between drug and polymer, the correct ASD-forming polymer needs to be selected carefully (Amponsah-Efah et al., 2021; Pöstges et al., 2023; Warren et al., 2010). Monschke et al. described Eudragit® L 100–55 (EL 100–55) as excellent matrix polymer for a ketoconazole ASD, as a high initial dissolution rate, followed by supersaturation stabilization for the entire observation period of 180 min, was observed in pH 6.8 medium (Monschke et al., 2021).
However, in some cases one single polymer cannot fulfill both requirements for being an optimal supersaturating ASD-forming polymer, as either a high initial drug release or an effective precipitation inhibition is missing upon dissolution (Alonzo et al., 2011; Xie and Taylor, 2016). Thus as alternative to the selection of one polymer, polymer combinations for forming ternary ASDs can be utilized, leading to synergistic enhancement in terms of solubility enhancement and supersaturation stabilization (Prasad et al., 2014; Zecevic et al., 2014).
By utilizing two polymers, a homogeneous or a heterogeneous ternary ASD can be obtained, depending on mixing of both polymers (Lyu et al., 2005). As the mixing of polymers can impact the intermolecular interactions between the polymers and the drugs, the phase behavior needs to be investigated (Butreddy, 2022; Sarpal et al., 2020). Monschke and Wagner processed a ternary ASD, consisting of polyvinyl alcohol (PVA) and hydroxypropylmethylcellulose acetate succinate (HPMCAS), and detected two glass transition temperatures (Tgs), representing PVA-richer and HPMCAS-richer phases, thus these polymers were not miscible or only partially miscible. Although the processed ternary ASD outperformed the corresponding binary ASDs, the ternary ASD did not exhibit the full potential of each polymer, as the dissolution of the fast dissolving binary PVA ASD with externally added HPMCAS as precipitation inhibitor demonstrated superior dissolution performance (Monschke and Wagner, 2020). Instead, PVA was reported to be miscible with copovidone (PVP-VA) (30:70 polymer mass ratio). The ternary ASD using itraconazole as drug demonstrated superior dissolution performance compared to the corresponding binary ASDs (Wlodarski et al., 2018). Synergistic interplay of miscible polymer blends in terms of solubility enhancement was also observed for spray-dried ternary griseofulvin ASDs, using hydroxypropylmethylcellulose and methacrylic acid copolymers (Ohyagi et al., 2017).
Although the importance of homogeneous and miscible polymer blends were emphasized in several studies (Higashi et al., 2015; Marks et al., 2014; Thompson et al., 2023), ternary ASD processing was also shown to be beneficial when immiscible polymer blends were utilized (Hörmann et al., 2018; Six et al., 2004; Yang et al., 2013).
Yang et al. processed ternary ASDs, consisting of felodipine, Eudragit® E PO, and PVP-VA and observed phase-separation phenomena, as the polymers were not miscible and felodipine was preferably distributed within the PVA-VA-richer domains. However, compared to the binary ASD formulations, the ternary ASD (10% drug load) benefited in dissolution and stability due to overall improvement of physical properties, as felodipine demonstrated high solubility in the hydrophilic PVP-VA for exhibiting drug-polymer interactions, and Eudragit® E PO reduced the hygroscopicity of the formulation. Thus, for mechanistic understanding of the improved stability and dissolution phenomena, the investigation of the drug distribution in the phase-separated ASD was demonstrated to be decisive.
In a previous study of our workgroup, manufacturing-dependent polymer mixing was demonstrated. Placebo and ternary celecoxib (CXB) ASD formulations using the polymers EL 100–55 and hydroxypropyl cellulose (HPC)-SSL (50:50 polymer mass ratio) were prepared via vacuum compression molding (VCM) and hot-melt extrusion (HME). During VCM processing, the mixture was only exposed to heat, while the ASD preparation using HME occurred under heat and additional shear forces through the kneading elements. While VCM did not lead to a homogeneous polymer mixture, a single-phased system was obtained after the HME process. It was assumed that shear forces through HME were required for homogeneous mixing of these polymers. As a homogeneous and intimate polymer mixture was important for the synergistic enhancement of the supersaturation of the drug, the determination of the phase homogeneity and interactions of the polymers was highly relevant (Pöstges et al., 2022).
The aim of this current study was to extend the insights of the previous study and to gain deeper understanding of the impact of phase homogeneity/ interaction on dissolution performances of ternary EL 100–55: HPC ASDs, using efavirenz (EFV) as alternative model drug. Next to HPC-SSL (molecular weight of 40,000 g/mol), we introduced HPC-UL (molecular weight of 20,000 g/mol) as additional polymer partner of EL 100–55. Therefore, not only the dependence of the manufacturing technique (VCM and HME), but also the selection of the HPC grade on the solid-state and dissolution performance of ternary ASDs was investigated and evaluated. Additionally, the drug distribution within the phase-separated ternary ASDs and the underlying mechanism were determined, since the knowledge about the drug localization would provide mechanistic insights regarding solid-state solubilities and dissolution processes.
Download the full article as PDF here Phase homogeneity in ternary amorphous solid dispersions and its impact on solubility, dissolution and supersaturation – Influence of processing and hydroxypropyl cellulose grade
or read it here
Materials
HPC-SSL and HPC-UL were supplied by Nippon Soda Co., Ltd. (Tokyo, Japan). EL 100–55 was kindly donated by Evonik (Darmstadt, Germany). The model drug EFV was purchased from Swapnroop Drugs&Pharmaceuticals (Aurangabad, India). Di‑sodium hydrogen phosphate dihydrate and sodium dihydrogen phosphate dihydrate for preparing the dissolution medium were obtained from Th. Geyer (Renningen, Germany).
Florian Pöstges, Jonas Lenhart, Edmont Stoyanov, Dominique J. Lunter, Karl G. Wagner, Phase homogeneity in ternary amorphous solid dispersions and its impact on solubility, dissolution and supersaturation – Influence of processing and hydroxypropyl cellulose grade, International Journal of Pharmaceutics: X, Volume 6, 2023, 100222, ISSN 2590-1567, https://doi.org/10.1016/j.ijpx.2023.100222.
Watch our free webinar “Hot Melt Extrusion (HME) – First webinar of the Amorphous Solid Dispersion (ASD) Webinar Series” here:


