QbD-driven development of phospholipid-embedded lipidic nanocarriers of raloxifene: extensive in vitro and in vivo evaluation studies
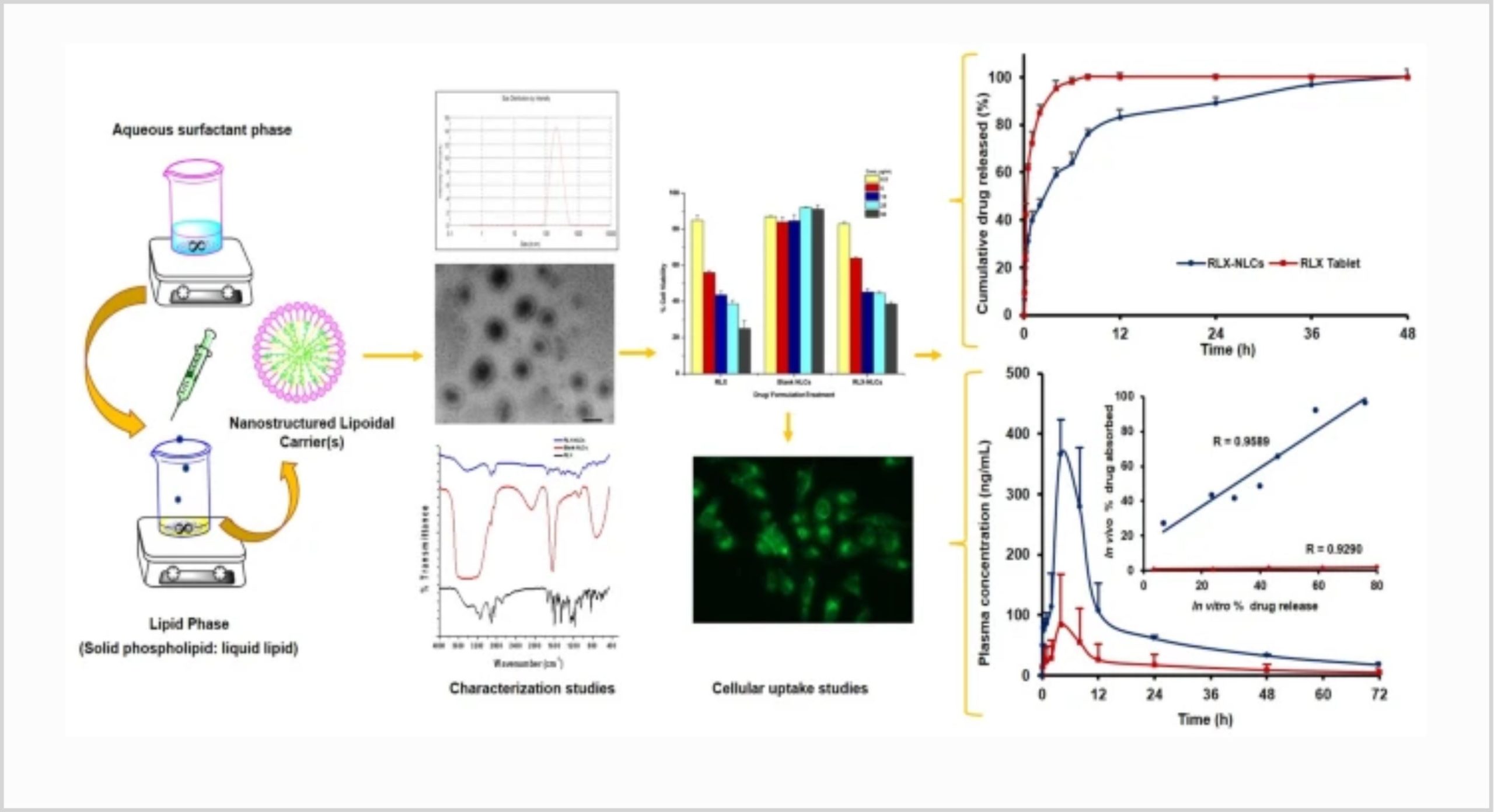
Raloxifene (RLX) is popularly indicated in treatment of osteoporosis and prevention of breast cancer. Owing to its poor aqueous solubility, high pre-systemic metabolism, intestinal glucuronidation, and P-glycoprotein (P-gp) efflux, however, it demonstrates low (< 2%) and inconsistent oral bioavailability. The current work, Quality by Design (QbD)-driven development of phospholipid-embedded nanostructured lipidic carriers (NLCs) of RLX, accordingly, was undertaken to potentiate its lymphatic uptake, augment oral bioavailability, and possibly reduce drug dosage. Factor screening and failure mode effect analysis (FMEA) studies were performed to delineate high-risk factors using solid lipid (glyceryl monostearate), liquid lipid (vitamin E), and surfactant (Tween 80). Response surface optimization studies were performed employing the Box-Behnken design.
Mathematical and graphical methods were adopted to embark upon the selection of optimized NLCs with various critical quality attributes (CQAs) of mean particle size as 186 nm, zeta potential of − 23.6 mV, entrapment efficiency of 80.09%, and cumulative drug release at 12 h of 83.87%. The DSC and FTIR studies, conducted on optimized NLCs, indicated successful entrapment of drug into the lipid matrix. In vitro drug release studies demonstrated Fickian diffusion mechanism. In vivo pharmacokinetic studies in rats construed significant improvement in AUC0–72 h (4.48-folds) and in Cmax (5.11-folds), unequivocally indicating markedly superior (p < 0.001) oral bioavailability of RLX-NLCs vis-à-vis marketed tablet formulation.
Subsequently, level “A” in vitro/in vivo correlation (IVIVC) was also successfully attempted between the percentages of in vitro drug dissolved and of in vivo drug absorbed at the matching time points. In vitro cytotoxicity and cellular uptake studies also corroborated higher efficacy and successful localization of coumarin-6-loaded NLCs into MG-63 cells through microfluidic channels.
Read more here
Pant, A., Sharma, G., Saini, S. et al. QbD-driven development of phospholipid-embedded lipidic nanocarriers of raloxifene: extensive in vitro and in vivo evaluation studies. Drug Deliv. and Transl. Res. (2023). https://doi.org/10.1007/s13346-023-01427-3
See the webinar:
“Rational Selection of Cyclodextrins for the Solubilization of Poorly Soluble Oral Drugs”, 8. November 2023:
Get more information & register here for free:


