Use of polyvinyl alcohol (PVA) to overcome challenges in ophthalmic formulations

Successful ophthalmic drug formulations rely on the selection of the correct polymer to ensure the desired characteristics such as retention in the eye cavity and solubility. One example of such an excipient is polyvinyl alcohol (PVA) which offers many advantages for ophthalmic drug formulations including eye drops, suspensions, and gels. It is a biocompatible synthetic polymer produced by the polymerization of vinyl acetate and partial hydrolysis of the resulting esterified polymer. PVA is recognized as safe (GRAS) by the US Food and Drug Administration (FDA) , does not have any immunogenic effects, and its long-term use has been demonstrated in many different formulations including oral, topical and ophthalmic.
ADVANTAGES OF PVA FOR OPHTHALMIC FORMULATION
PVA offers many advantages for ophthalmic formations including eye drops and hydrogels. It is water soluble and has a narrow range of viscosity and a high degree of swelling, offering the precise viscosity needed for formulations to remain in the eye cavity. Scientific evidence and long-term use confirm the physiological compatibility and safety of PVA, while a variety of grades allows selection of the exact properties needed for specific formulations.
Its physicochemical properties make PVA especially well suitable for the application in ophthalmic drug delivery systems including:
- The ability to form a transparent solution which is essential for medications administered to the eye
- High adhesion and high correlation properties, which enable retention in the eye cavity
- Suitability for lubricating eye drops
- The ability to act as an inhibitor of crystallization which helps retain solubility of the API throughout storage of the dosage form
SELECTING THE HYDROLYSIS GRADE OF PVA BASED ON VISCOSITY
As a synthetic polymer, PVA offers batch-to-batch consistency and is available in different grades defined by viscosity, hydrolysis and the microbial load (total aerobic bacteria, total yeast and total mold count), which should be specified by the manufacturer. These grades come with different physicochemical properties, making them suitable for different applications.
Lower viscosity grades of PVA can be used for lubricant activity, to enhance API solubility and as inhibitors of crystallization. If the formulation is a suspension or gel, a high viscosity grade PVA is more suitable and can be used as a thickener or viscosity enhancer. Different hydrolysis grades of PVA, which refer to the amount of residual, unhydrolyzed acetate groups within the polymer chain, also affect the polymer performance. Higher hydrolysis grades improve tensile strength of the hydrogel and provide a stronger gel scaffold through H-bonds while lower hydrolysis grades might be a better choice for drug delivery of poorly water-soluble APIs. However, since some pharmacopoeias restrict the hydrolysis grade variation, there is limited flexibility in this aspect.
Another important consideration when using PVA is the possible presence of crotonaldehyde, which is a strong eye irritant and a process impurity generated during synthesis. While there is no information in the pharmacopeia regarding the limit of this impurity, given its toxic nature, it is important that this impurity content is known and controlled. Due to stringent regulatory requirements in this segment, a multi-compendial product and regulatory and documentation support by the manufacturer is essential to facilitate regulatory submission. We specify low crotonaldehyde limits for PVAs.
PVA SOLUTION PREPARATION FOR OPHTHALMIC APPLICATIONS
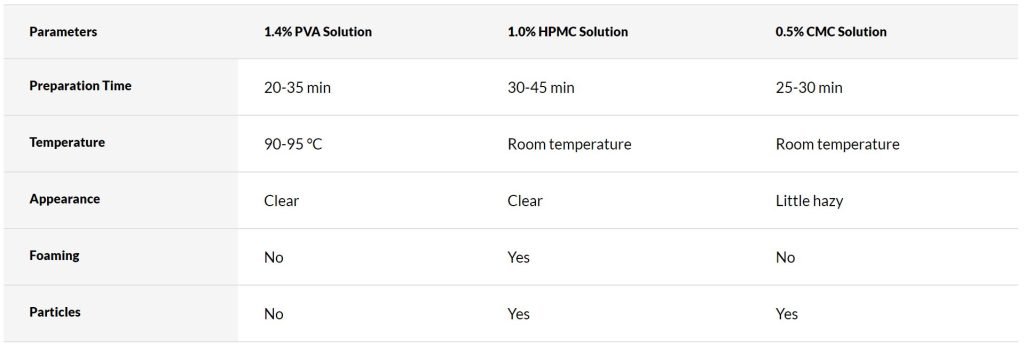
Table 1 compares the preparation, appearance, and presence of foam and particles in solutions of three different polymers commonly used in ophthalmic preparations: PVA, and semi-synthetic options such as hydroxypropylmethylcellulose (HPMC) and carboxymethyl cellulose (CMC). While a higher temperature is recommended for PVA dissolution, the result will be a clear, particle free solution without foam. HPMC and CMC can be dissolved at room temperature, but it may be challenging to obtain particle-free solutions, and foaming was observed during HPMC solution preparation.
Read more
Source: Merck, Use of polyvinyl alcohol (PVA) to overcome challenges in ophthalmic formulations, https://www.sigmaaldrich.com/DE/en/technical-documents/technical-article/pharmaceutical-and-biopharmaceutical-manufacturing/liquid-formulation-strategies/polyvinyl-alcohol-pva-in-opthalmic-formulations
Read also the following articles about Ophthalmics and PVA by Merck:
- Overcoming Challenges in Ophthalmic Formulations through Polymer Selection – A Closer Look at Polyvinyl Alcohol
- Ophthalmics Process by Merck – Keep a close eye on quality and safety


