Preformulation screening of lipids using solubility parameter concept in conjunction with experimental research to develop ceftriaxone loaded nanostructured lipid carriers
Abstract
Development of ceftriaxone loaded nanostructured lipid carriers to increase permeability of ceftriaxone across uninflamed meninges after parenteral administration. Lipids were selected by theoretical and experimental techniques and optimization of NLCs done by response surface methodology using Box-Behnken design. The Δδt for glyceryl monostearate and Capryol90 were 4.39 and 2.92 respectively. The drug had maximum solubility of 0.175% (w/w) in glycerol monostearate and 2.56g of Capryol90 dissolved 10mg of drug. The binary mixture consisted of glyceryl monostearate and Capryol90 in a ratio of 70:30. The optimized NLCs particle size was 130.54nm, polydispersity index 0.28, % entrapment efficiency 44.32%, zeta potential -29.05mV, and % drug loading 8.10%. In vitro permeability of ceftriaxone loaded NLCs was 5.06×10-6 cm/s; evidently, the NLCs pervaded through uninflamed meninges, which, was further confirmed from in vivo biodistribution studies. The ratio of drug concentration between brain and plasma for ceftriaxone loaded NLCs was 0.29 and that for ceftriaxone solution was 0.02. With 44.32% entrapment of the drug in NLCs the biodistribution of ceftriaxone was enhanced 7.9 times compared with that of ceftriaxone solution. DSC and XRD studies revealed formation of imperfect crystalline NLCs. NLCs improved permeability of ceftriaxone through uninflamed meninges resulting in better management of CNS infections.
Introduction
The challenge associated with the delivery of drugs to central nervous system (CNS) is to surmount the problem of permeability through different barriers like blood brain barrier, blood-cerebrospinal barrier, and the different efflux system (Santaguida et al., 2006; Huttunen, Rautio, 2011). In case of CNS infections these barriers act peculiarly, the meninges inflame during the peak infection and the distorted meninges allows the free passage of the xenobiotics. The problem arises when the infection is on the verge of cure, the meninges start to repair itself and become uninflamed, hindering the penetration of antimicrobial drugs, resulting in drug resistance (Nau et al., 1993; Emmerson et al., 1985). This problem can be solved by increasing the amount of drug administration, but this may result in toxic side effects.
Ceftriaxone sodium is hydrophilic in nature and enlisted under the cephalosporin class of antibiotic, mainly used as a drug of choice for childhood diseases and bacterial meningitis for susceptible microorganisms (Mandell, Sande, 1996). When the infection is on the approach to cure, the meninges become uninflamed impeding the access of antimicrobial drugs to the brain. Therefore, due to low concentration of drug in cerebrospinal fluid (CSF) during uninflamed meninges cannot act against moderately or resistant bacterial infection, hence the dose has to be increased to maintain the minimum inhibition concentration of drug (Cherubin et al., 1989), which, may otherwise lead to multi-drug resistance leading to failure of the treatment strategy. The difficulty of ceftriaxone in the current formulation (solution) in crossing BBB is well known (Nau et al.,1993). This restriction associated with ceftriaxone solutions has lead the researchers to reformulate it into such a formulation which allow the drug permeation through uninflamed meninges. As the uninflamed meninges are highly lipophilic in nature and the drug was hydrophilic in nature so it was necessary to design a lipophilic carrier having sufficient space to entrap and enhance the permeability of hydrophilic drug.
Nanostructure lipid carriers (NLCs) may be a suitable dosage form that improves the penetration of the therapeutic agent across the blood brain barrier (BBB) by influencing the tight junction or by the endocytosis process (Padhi, Mazumder, Bisth, 2019; Salunkhe, et al., 2015). NLCs enhances the retention time of drug and maintain its required concentration in the brain for effective and rational treatment of infectious diseases of CNS.
Lipid nanoparticles comprised of solid lipid nanoparticles and nanostructured lipid carriers belonging to first and second generation respectively (Tajes et al., 2014; Gartziandia et al., 2015; Almousallam, Moia, Zhu, 2015) The solid lipid nanoparticles (SLN) is mainly composed of biodegradable solid lipids whereas NLCs had liquid lipids in addition. The incorporation of a small amount of liquid lipids in solid lipids results in the formation of the imperfect crystalline structure of NLCs, which in turn increases its drug loading capability compared to SLN which has crystalline and rigid structure (Neves, Queiroz, Reis, 2016). Therefore, NLCs are suitable for carrying both hydrophilic and lipophilic drugs (Kasongo et al., 2011; Kasongo, Müller, Walker, 2012)
The purpose of this research is to develop parenteral nanostructured lipid carriers of ceftriaxone sodium to enhance the permeability of the same, thus increasing the delivery of the drug to CNS. NLCs carriers due to their lipophilic nature can easily cross the BBB and are able to deliver the hydrophilic drug to the brain. Preformulation studies were carried out to select the suitable solid lipid and liquid lipid. Ceftriaxone loaded NLCs were formulated and optimized using Box Behnken design. In vitro permeability studies were carried out by using parallel artificial membrane permeability assay and biodistribution studies in rat’s brain were performed. X-ray diffraction and thermal analysis gave the structural interpretation of the optimized formulation (Shah et al.,2019).
Material And Methods
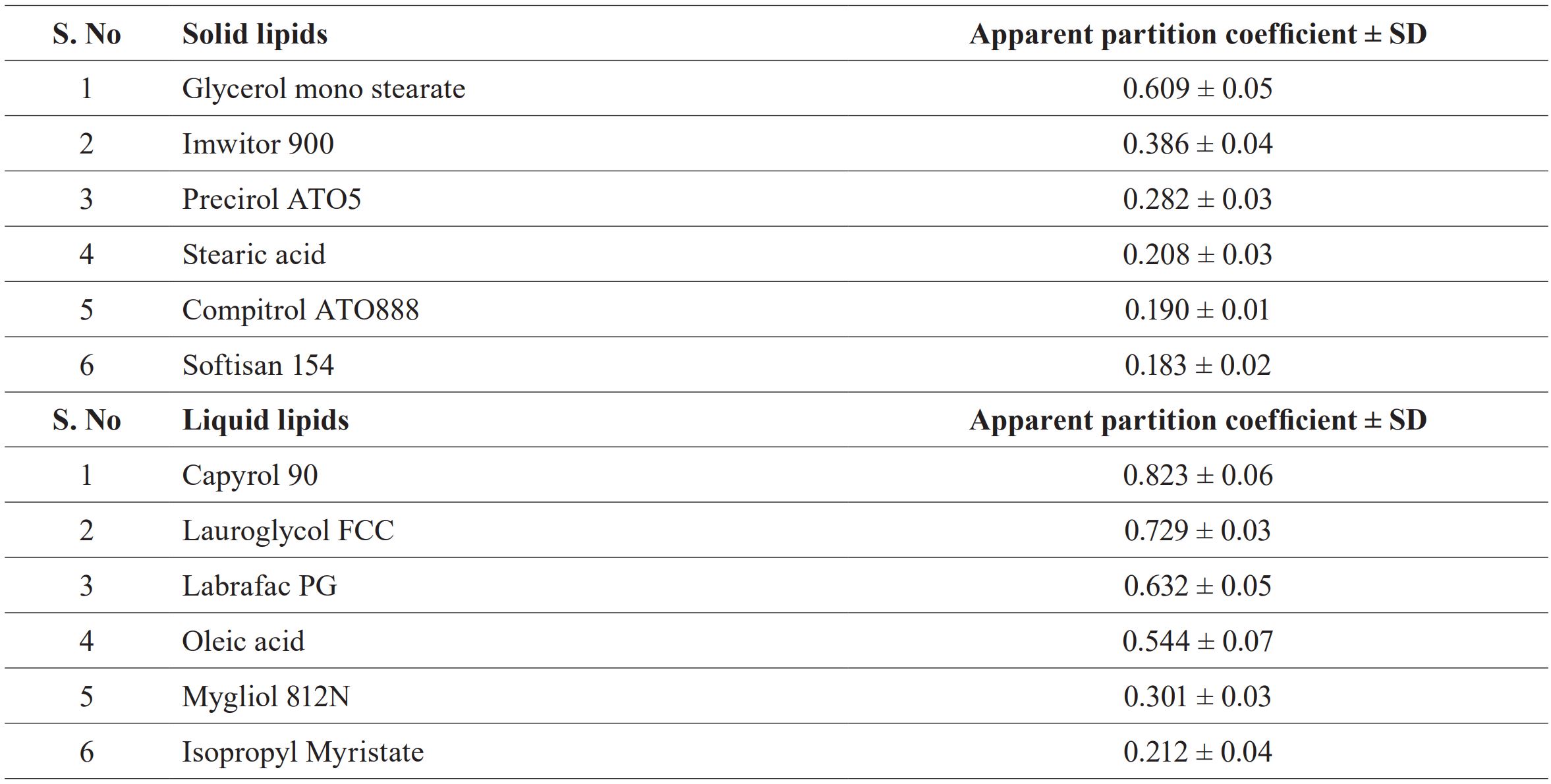
Ceftriaxone (disodium salt) was obtained as a gift sample from Cipla limited, Himachal Pradesh (India). Solid lipids glyceryl monostearate (58 to 59ºC), Stearic acid (MP: 67to72ºC), Oleic Acid, Isopropyl myristate, and Tween 80 were obtained from CDH Pvt. Ltd. Compritol 888 pellets (69-74ºC), Precirol ATO 5 (52-56ºC), Capryol90 (propylene glycol monocaprylate) Lauroglycol FCC(propylene glycol monolaurate (TypeI)), Labrafac PG (propylene glycol dicaprylate /dicaprate) gifted by Gattefosse India Pvt. Ltd., Softisan 154 (53-58ºC), Imwitor 900(F)P (54-64ºC), Miglyol 812N (Caprylic/ Capric Triglyceride) were gifted by IOI Oleo GmbH. Porcine Polar Brain Lipid (PBL) was obtained from Sigma Aldrich, India. Distilled water was prepared using a double distillation unit. All other ingredients used were of analytical grade and purchased from CDH Pvt. Ltd. India.
Download the full article as PDF here Preformulation screening of lipids using solubility parameter concept in conjunction with experimental research to develop ceftriaxone loaded nanostructured lipid carriers
or read it here
Swarupanjali Padhi, Rupa Mazumder, Shradha Bisht, Preformulation screening of lipids using solubility parameter concept in conjunction with experimental research to develop ceftriaxone loaded nanostructured lipid carriers, Braz. J. Pharm. Sci. 59 • 2023 • https://doi.org/10.1590/s2175-97902023e21308
Read also more on Sodium Stearyl Fumarate as a pharmaceutical excipient here:


