Quality by design (QbD) commended exploration of bosutinib loaded lipid nanocarriers for food effect attenuation and bioavailability enhancement in breast cancer
Abstract
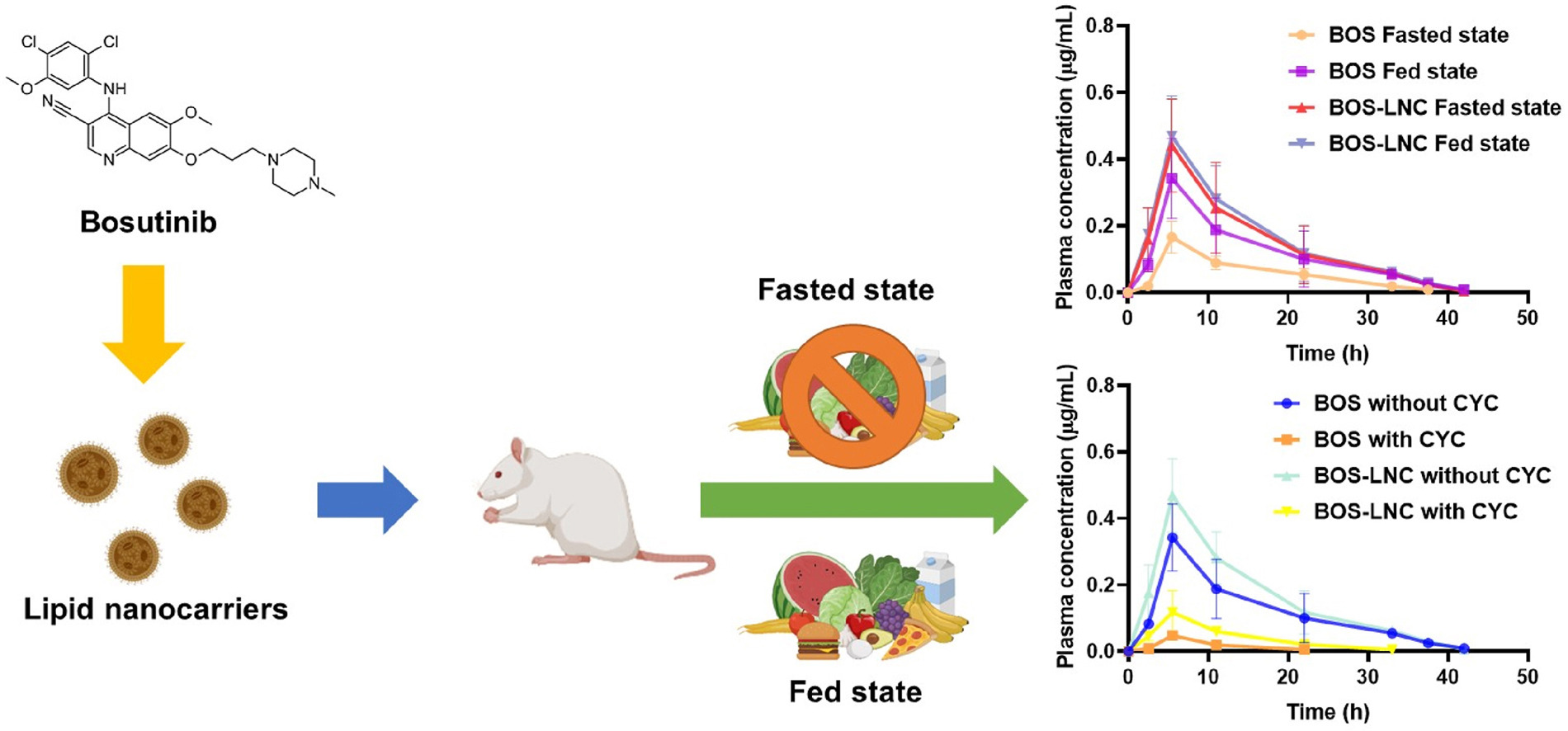
Bosutinib (BOS), a BCS class IV drug suffers from poor aqueous solubility and bioavailability along with significant food effect. Lipid nanocarriers (LNC) have the potential to circumvent bioavailability related issues associated with hydrophobic moieties. In this endeavor, we explore the potential of novel lipid nanocarriers to circumvent the bioavailability pitfalls, while minimizing food effect and pharmacokinetic variability. Quality by design and multi-variate analysis was adopted for the optimization of various process parameters during nanoparticle fabrication. The optimized particle size, polydispersity index (PDI) and zeta potential were found to be 131.7 nm, 0.105 and −9.29 mV respectively. Ex vivo studies revealed improved intestinal permeation of LNC. Enhancements in cytotoxicity, ROS generation and apoptosis were observed against MCF-7 cells that could be attributed to elevated intracellular drug accumulation using LNC. Pharmacokinetic studies revealed a 2.6-fold increase in the bioavailability of BOS during fasted state and a 1.36-fold increase during fed state when BOS was administered via LNC, indicating superior oral bioavailability. Cmax fast/fed was found to be 0.94 while, AUC fast/fed was 0.9258 which was close to 1, indicating attenuation of the food effect by BOS LNC. Therefore, LNCs can serve as promising delivery carriers for attenuation of food effect during breast cancer therapy.
Introduction
Aqueous solubility of therapeutic agent(s) plays an important role in governing the rate and extent of drug absorption, and consequential bioavailability. Drug discovery and development data indicate that majority of newly discovered drugs show a drawback of poor aqueous solubility. Such therapeutic agents pose challenges to achieve desirable pharmacokinetic profile in vivo. Bosutinib, a dual tyrosine kinase inhibitor that is used for the treatment of Philadelphia chromosome-positive chronic myelogenous leukemia (CML) is a BCS class IV drug that exhibits poor solubility in physiological fluids and low permeability across the gastrointestinal tract (GIT) when administered orally. As a result, the oral dose of bosutinib shows limited bioavailability [1]. Therefore, there is a need to develop appropriate formulation strategies to improve the bioavailability of bosutinib. Literature reveals that lipid-based nanoparticulate drug delivery systems can improve the bioavailability of poorly water-soluble drugs by promoting lymphatic transport [2]. Orally administered lipid nanoparticles undergo hydrolysis and transform into micelles. The resultant micelles subsequently transported into the lymphatic circulation via chylomicrons. Studies revealed that lipid nanoparticles improved the bioavailability of a poorly soluble drug, raloxifene when administered orally [3]. Since bosutinib is a poorly soluble drug, such an absorption mechanism can be explored to improve its oral bioavailability and consequential therapeutic efficacy. In addition to low oral bioavailability, the other major drawback associated with bosutinib absorption is fast-fed variability (see Table 1).
Fast-fed variability can be defined as an alteration in drug absorption based on the presence or absence of food. Since physiological parameters such as gastric emptying time, gastrointestinal pH, splanchnic blood flow and bile flow are influenced by intake of food, drug absorption can be altered due to the presence of food. The extent of alteration in turn depends on the physico-chemical properties of drugs. Bosutinib, being a low soluble and low permeable drug, fast-fed variability significantly affects its absorption, thereby showing variable pharmacokinetic behaviour [4]. As a result, the plasma-drug concentration may deviate from the therapeutic window thereby causing compromised therapeutic efficacy. Further, fast-fed variability causes faulty drug dosing, intra- and inter-individual variability thereby leading to serious adverse consequences. Hence, there is a need for minimization of fast-fed variability using suitable drug delivery interventions so as to attain reproducible pharmacokinetics and consequential pharmacodynamic effect while minimizing the occurrence of adverse effects [5].
Various drug delivery strategies such as preparation of prodrugs, salt derivatives, micelles, solid dispersions, cyclodextrin complexes, lipid-based drug delivery systems have been explored thus far to improve bioavailability and to minimize fast-fed variability [[6], [7], [8]]. Lipid nanocarriers (LNC) have offered dual advantages such as improved bioavailability while causing minimization of fast-fed variability. These drug delivery vehicles are composed of a lipid matrix wherein drug molecules are dispersed in the amorphous form, thereby enabling higher entrapment of hydrophobic drugs [5]. Further, these systems help to protect drugs from external harsh environment (such as chemical or enzymatic degradation), offer sustained drug release that in turn help to maintain therapeutic concentration of bioactive molecules for longer duration. As a consequence, the frequency of drug administrations can be minimized while improving therapeutic efficacy [9]. Such a delivery system can also be explored for the delivery of cytotoxic drugs in the treatment of cancer.
Breast cancer is the most common type of cancer diagnosed in women. Its metastatic nature makes it one of the leading causes of death among women across the globe. BOS has been demonstrated to inhibit tumor progression and metastasis in the advanced breast tumors [10]. Based on aforementioned literature, we hypothesized that development of BOS LNC may help in improving absorption of BOS while minimizing fast-fed variability. This in turn enable uniform absorption while avoiding pharmacokinetic variability after oral administration. These characteristics of the delivery system may help to improve therapeutic efficacy of BOS in the treatment of breast cancer. To test this hypothesis, we fabricated BOS LNCs using QbD approach, performed in vitro cytotoxicity studies (in MCF-7 cell line), ex vivo intestinal permeability studies (in rat intestine) and in vivo pharmacokinetic studies (in Sprague Dawley rats).
Read more here
Materials
Bosutinib was obtained from MSN Laboratories Pvt Ltd Hyderabad, India. Pluronic F68 and Pluronic F127 were obtained from Sigma-Aldrich, India. Polysorbate (Tween® 80) was obtained from Avra chemicals, India. Glycerol monostearate (GMS), Stearic acid, Trimyristin, Trilaurin and Tripalmitin were procured from Tokyo Chemicals, India. Compritol ATO 888®, Capryol PGMC®, Labrafil® (LBF), Labrasol® (LBSL), Lauroglycol® (LGL), Capmul MCM8® were obtained from Gattefosse, Saint-Priest Cedex, France
Paras Famta, Saurabh Shah, Ganesh Vambhurkar, Dadi A. Srinivasarao, Deepkumar Bagasariya, Kondasingh Charan Kumar, Nusrat Begum, Anamika Sharma, Syed Shahrukh, Naitik Jain, Gurpreet Singh, Sajja Bhanu Prasad, Akshay Shinde, Dharmendra Kumar Khatri, Saurabh Srivastava, Quality by design (QbD) commended exploration of bosutinib loaded lipid nanocarriers for food effect attenuation and bioavailability enhancement in breast cancer, Journal of Drug Delivery Science and Technology, Volume 90, 2023, 105180, ISSN 1773-2247, https://doi.org/10.1016/j.jddst.2023.105180.
See our record QbD webinar by Dr. Pöllinger here:


