Data-driven approach to mitigate quality impact of hygroscopic pharmaceutical raw materials throughout the supply chain
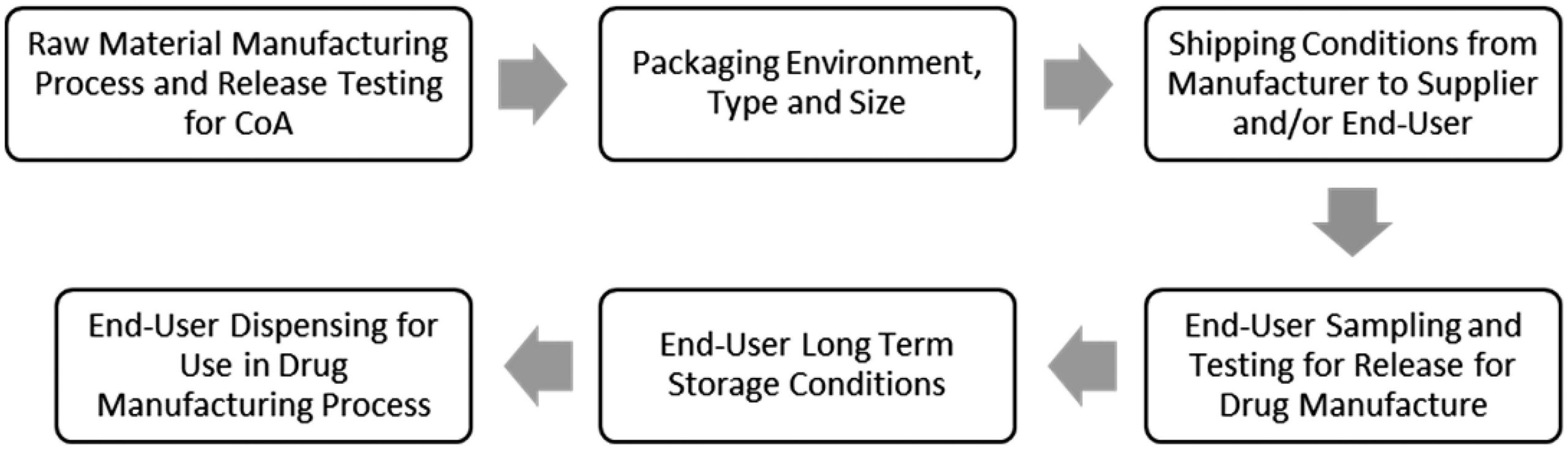
The challenges of working with hygroscopic pharmaceutical raw materials can have a significant impact on the industry’s ability to make high-quality medicines. In order to mitigate the impact to the manufacturing process or product quality it is critical to understand the hygroscopicity of the raw materials across the entire supply chain so that the proper management strategies can be implemented, from the raw material manufacturing to the use of the raw material in the drug manufacturing process. Employing suitable controls protects these materials from physical and chemical changes due to moisture uptake such as caking or hydrolysis. We have developed a fit for purpose and data-driven approach to hygroscopicity classifications of over 200 commonly used chemicals, excipients, media and resins in drug manufacturing processes. Dynamic vapor sorption data is presented with supporting thermal gravimetric analysis and X-ray powder diffraction data where pertinent. Approximately 60% of all raw materials tested were determined to be hygroscopic. Strategies for applying this data to reduce the potential impact of hygroscopic materials on the manufacturing of pharmaceuticals are discussed with examples.
Introduction
High quality chemicals, excipients, cell culture media and chromatography resins are crucial to successful manufacturing of drug substance (DS) and drug product (DP) in the pharmaceutical industry. To ensure that these raw materials are suitable for DS and DP manufacturing, quality control measures are required throughout the supply chain during the manufacturing and packaging of these raw materials as well as shipping, sampling, long-term storage and dispensing (Figure 1). One attribute of these raw materials that must be considered throughout this process is hygroscopicity or the propensity for the material to readily take up and retain moisture. An increase in moisture content in a raw material may cause physical (e.g. caking, deliquescence, color change, crystal form change), chemical (e.g. hydrolysis) or purity (e.g. assay, water content) changes rendering the material unsuitable for the drug manufacturing process. Therefore, it is important during drug development to have a clear picture of each raw material’s hygroscopicity and the risks associated with moisture uptake to the raw material attributes, the drug manufacturing process, and ultimately to the ability to meet the target product profile. Hygroscopic raw materials with a risk of impact to the DS or DP need to be identified to enable mitigations to be put in place to avoid exposure to moisture and ensure product quality.

One common source of information for hygroscopicity is the raw material safety data sheets or product data sheet from the raw material manufacturer or supplier. However, this information is often contradictory, vague or not listed (which could mean the material is not hygroscopic or it is unknown) and no references are provided. Literature sources may offer a more detailed hygroscopicity analysis, however the analytical methods used to determine hygroscopicity may be variable between sources or not fit-for-purpose. Murikipudu et al determined the hygroscopicity of 30 inactive and 10 active pharmaceutical ingredients by 3 different methods showing that the hygroscopicity classification can vary with the method employed (Murikipudi et al. 2013). Additionally, most literature focuses on the hygroscopicity of excipients used for tableting and active pharmaceutical ingredients, and only those in powder form (Callahan et al. 1982; Visalakshi et al. 2005; Murikipudi et al. 2013). Included in this research are raw materials used in synthetic and biologic drug manufacturing which includes a wide variety of excipients, chemicals and media that may be in solid or liquid form. Similarly, different manufacturers (or different lots from the same manufacturer) of the same raw material may have variable hygroscopicity due to alterations in physical properties such as particle size, crystallinity or porosity (Bronlund and Paterson 2004; Sun 2011). To avoid these discrepancies and ensure the proper control of raw materials used in our processes we developed a data-driven hygroscopicity classification system, utilizing dynamic vapor sorption (DVS) analysis and the European Pharmacopeia hygroscopicity criteria, that is applicable to the temperature and humidity conditions the materials are exposed to throughout the supply chain. Thermal gravimetric analysis (TGA) and X-ray powder diffraction (XRPD) were used as supporting analytical techniques to ensure accurate interpretation of the DVS data. The results for over 200 raw materials are presented and discussed.
Utilizing the results of this data-driven approach, the hygroscopicity classifications were updated on all internal raw material specifications and general handling recommendations were made to ensure the proper management of all hygroscopic raw materials within our network. We then conducted individual risk assessments for each hygroscopic raw material to identify those materials at high risk of impacting a manufacturing process or drug product due to moisture uptake and implemented additional preventative mitigations where needed. These mitigations, such as establishing the appropriate raw material specification, collaborating with the supplier to ensure proper handling and storage of the material or even suggesting non-hygroscopic alternatives, are discussed with case studies to emphasize the importance of understanding raw material attributes such as hygroscopicity.
Materials and Methods
Over 200 raw materials were selected for analysis as they are commonly used in the drug manufacturing process as chemicals, excipients, media or resins. All raw materials were procured from certified commercial sources. Brand names are provided in the tables where available to help clarify the material grade and source.
Moisture sorption data to determine the hygroscopicity classification was collected using a DVS Endeavor (Surface Measurement Systems) dynamic vapor sorption (DVS) analyzer. Samples (∼100 mg) were evaluated in an aluminum pan by equilibrating at 30% RH (equilibration set to a dm/dt of 0.001% wt/min with a minimum of 10 min and maximum of 3 h), then an intermediate step at 65% RH (equilibration set to a dm/dt of 0.001% wt/min with a minimum of 10 min and maximum of 3 h) then the final hold at 80% RH (for 24 h) at 25 °C with a nitrogen flow rate of 500 sscm. Equilibrium was not always achieved during this final 24 h hold. The increase in mass from the end of the 30% RH step to the end of the 80% RH step was used to assign the hygroscopicity classification (‘H-1′ to ‘H-4′) according to Table 1 unless otherwise noted in the result tables. The qualitative term of ‘hygroscopic materials’ used in this manuscript includes materials classified as hygroscopic (H-3) or very hygroscopic (H-4). Since the end of the 30% RH step is the zero point (or baseline), any weight loss/gain recorded during the 30% RH step is additive to the weight gain at 80% RH. In some cases (evaporative liquids), the experiment was started at 80% RH as noted in the data tables to avoid evaporation at lower humidity.
Data supporting hygroscopicity classification of excipients
| Raw material | Crystallinity pre-DVS | Weight loss (% wt to Temp °C) | % Weight change (25 °C) | Crystallinity | Hygroscopicity classification | ||
|---|---|---|---|---|---|---|---|
| 30% RH (%) | 65% RH (%) | 80% RH (%) | Post-DVS | ||||
| β-Cyclodextrin | Crystalline | 12.5% to 150 | 2022-02-01 00:00:00 | 2022-05-01 00:00:00 | 2022-09-01 00:00:00 | Same | H-3 |
| Betadex Sulfobutyl Ether Sodium (Captisol®) | Amorphous | 9.4% to 150 | −0.2 | 2022-01-11 00:00:00 | 2022-08-24 00:00:00 | Liquid | H-4 |
| Calcium Acetate | Crystalline | 1.9% to 175 | −0.1 | 0.5 | 1.0 | Different | H-2 |
| Carboxymethylcellulose Sodium | Amorphous | 8.4% to 150 | 2022-06-01 00:00:00 | 2022-07-11 00:00:00 | 2022-03-22 00:00:00 | Same | H-4 |
| Carnauba Wax | Crystalline | 0.1% to 150 | 0.0 | 0.1 | 0.1 | Same | H-1 |
| Citric Acid (Lot 1) | Crystalline | 0.0% to 150 | 0, 0 | 0, 0 | 16.6, 2.9 | Liquid, Same | H-4 |
| Citric Acid (Lot 2) | Crystalline | 0.0% to 150 | 0, 0 | 0, 0 | 45.9, 10.4 | Liquid, Liquid | H-4 |
| Colloidal Silicon Dioxide | Amorphous | 0.2% to 150 | 0.0 | 1.0 | 2022-09-02 00:00:00 | Same | H-3 |
| Copovidone (Kollidon VA 64) | Amorphous | 3.6% to 150 | 0.0 | 2022-07-08 00:00:00 | 2022-04-17 00:00:00 | Liquid | H-4 |
| Croscarmellose Sodium (Ac-Di-Sol) | Amorphous | 7.3% to 175 | −0.4 | 2022-04-07 00:00:00 | 2022-06-14 00:00:00 | Same | H-3 |
| Crospovidone (Polyplasdone XL) | Amorphous | 6.8% to 125 | 2022-04-02 00:00:00 | 14.0 | 2022-09-23 00:00:00 | Same | H-4 |
| Dicalcium Phosphate | Crystalline | 0.2% to 175 | 0.0 | 0.0 | 0.1 | Same | H-1 |
| Fumaric Acid | Crystalline | 0.0% to 150 | 0.0 | 0.0 | 0.0 | Same | H-1 |
| Glyceryl Dibehenate (Compritol® 888 ATO) | Crystalline | 0.6% to 150 | 0.0 | 0.4 | 0.8 | Same | H-2 |
| Hydroxypropyl Methylcellulose (MethocelTM K100LVCR) | Amorphous | 2.9% to 150 | −0.4 | 2022-09-05 00:00:00 | 11.0 | Same | H-3 |
| Hydroxypropyl Methylcellulose (MethocelTM K100MCR) | Amorphous | 2.9% to 150 | 0.0 | 2022-09-05 00:00:00 | 11.0 | Same | H-3 |
| Hydroxypropyl Methylcellulose (MethocelTM K15MCR) | Amorphous | 2.6% to 150 | 0.1 | 2022-03-07 00:00:00 | 2022-03-12 00:00:00 | Same | H-3 |
| Hydroxypropyl Methylcellulose (MethocelTM K3LV) | Amorphous | 2.4% to 150 | −0.2 | 5.0 | 2022-05-10 00:00:00 | Same | H-3 |
| Hydroxypropyl Methylcellulose (MethocelTM K4MCR) | Amorphous | 4.0% to 150 | −0.5 | 7.0 | 2022-01-12 00:00:00 | Same | H-3 |
| Hydroxypropyl Methylcellulose Acetate Succinate | Amorphous | 1.4% to 150 | 0.1 | 2022-06-02 00:00:00 | 2022-06-04 00:00:00 | Same | H-3 |
| Hydroxypropylcellulose (Klucel EXF) | Amorphous | 1.2% to 100 | 0.0 | 2022-08-04 00:00:00 | 2022-04-09 00:00:00 | Same | H-3 |
| Indigo Carmine (Acid Blue 74) | Crystalline | 5.6% to 150 | 0.2 | 2022-03-01 00:00:00 | 8.0 | Same | H-3 |
| Lactose (SheffieldTM DT) | Crystalline | 0.5% to 150 | 0.0 | 0.0 | 0.5 | Same | H-2 |
| Magnesium Stearate (HyQual®) | Crystalline | 3.6% to 125 | 0.0 | 0.1 | 0.2 | Same | H-1 |
| Maize Starch | Crystalline | 7.9%, 11.3% to 175 | 0, −2.0 | 4.2, 3.2 | 8.0, 6.2 | Same, Same | H-3 |
| Maltodextrin | Amorphous | 6.4% to 175 | −0.2 | 2022-07-04 00:00:00 | 2022-04-10 00:00:00 | Same | H-3 |
| Mannitol | Crystalline | 0.1% to 175 | 0, 0 | 0, 0.1 | 0.1, 0.2 | Same, Same | H-2 |
| Microcrystalline Cellulose (Avicel® PH-101) | Crystalline | 4.0% to 125 | −0.1 | 2022-08-02 00:00:00 | 5.0 | Same | H-3 |
| Microcrystalline Cellulose (Avicel® PH-102) | Crystalline | 3.8% to 150 | −0.1 | 2022-07-02 00:00:00 | 2022-09-04 00:00:00 | Same | H-3 |
| Microcrystalline Cellulose (Avicel® PH-200) | Crystalline | 3.6% to 150 | −0.4 | 2022-01-03 00:00:00 | 2022-04-05 00:00:00 | Same | H-3 |
| Myo-Inositol | Crystalline | 0.0% to 150 | 0.0 | 0.0 | 0.0 | Same | H-1 |
| N-Lauroylsarcosine sodium (Sarkosyl NL) | Crystalline | 0.8% to 125 | 3.0 | 2022-01-02 00:00:00 | 2022-04-03 00:00:00 | Amorphous | H-3 |
| Opadry® II | Crystalline | 1.3% to 150 | −0.3 | 2.0 | 2022-02-04 00:00:00 | Same | H-3 |
| Opadry® II Blue | Crystalline | 1.7% to 175 | −0.4 | 2.0 | 2022-09-03 00:00:00 | Same | H-3 |
| Opadry® II Clear | Crystalline | 1.6% to 150 | −0.2 | 2022-07-02 00:00:00 | 2022-07-05 00:00:00 | Same | H-3 |
| Opadry® II White | Crystalline | 9.5% to 175 | −0.4 | 2022-05-01 00:00:00 | 2022-07-02 00:00:00 | Same | H-3 |
| Opadry® II Yellow | Crystalline | 1.0% to 150 | 0.0 | 2022-05-02 00:00:00 | 2022-04-04 00:00:00 | Same | H-3 |
| Pentetic Acid | Crystalline | NA | 0.0 | 0.0 | 0.0 | Same | H-1 |
| Poloxamer 188 (Kolliphor® P 188) | Crystalline | 0.3% to 150 | −0.1 | 0.6 | 2022-02-01 00:00:00 | Same | H-2 |
| Potassium Phosphate Dibasic | Crystalline | 0.0% to 200 | 0.5, 1.6 | 18.8, 16.9 | 99.3, 108.5 | Liquid, Liquid | H-4 |
| Potassium Phosphate Monobasic | Crystalline | 0.0% to 200 | 0, 0 | 0, 0 | 0, 0 | Same, Same | H-1 |
| Povidone (Plasdone K-29/32) | Amorphous | 5.9% to 125 | 2022-03-03 00:00:00 | 2022-01-14 00:00:00 | 2022-02-27 00:00:00 | Liquid | H-4 |
| Sodium Dodecyl Sulfate | Crystalline | NA | 0.0 | 0.2 | 2022-02-02 00:00:00 | Same | H-3 |
| Sodium Phosphate Dibasic | Crystalline | 0.3% to 150 | 0.0 | 2022-07-03 00:00:00 | 2022-06-26 00:00:00 | Different | H-4 |
| Sodium Starch Glycolate (Explotab®) | Amorphous | 8.0% to 150 | 0.0 | 2022-04-07 00:00:00 | 20.0 | Same | H-4 |
| Sodium Stearyl Fumarate (PRUV®) | Crystalline | 3.0% to 150 | 0.0 | 0.0 | 0.0 | Same | H-1 |
| Soluplus® | Amorphous | 2.7% to 175 | −0.5 | 2022-08-03 00:00:00 | 2022-06-09 00:00:00 | Same | H-3 |
| Stearic Acid | Crystalline | 0.0% to 125 | 0.0 | 0.0 | 0.0 | Same | H-1 |
| Succinic Acid | Crystalline | 0.0% to 125 | 0.0 | 0.0 | 0.1 | Same | H-1 |
| Sucrose | Crystalline | 0.0% to 150 | 0.0 | 0.0 | 0.0 | Same | H-1 |
Download the full research paper as PDF: Data-driven approach to mitigate quality impact of hygroscopic pharmaceutical raw materials throughout the supply chain
(2022) Data-driven approach to mitigate quality impact of hygroscopic pharmaceutical raw materials throughout the supply chain, Pharmaceutical Development and Technology, DOI: 10.1080/10837450.2022.2084105
Read more on Sodium Stearyl Fumarate as a pharmaceutical excipient here:


