A Retrospective Analysis of Polymer Selection Using Solvent Casting: Formulation and DoE Optimization of the Amorphous Solid Dispersion of Amoxicillin Trihydrate by a Spray Drying Method
Abstract
Background. Amoxicillin trihydrate possesses poor solubility, compressibility, and flow behavior. Amorphous solid dispersion prepared by spray drying could solve all three problems at the same time. Objective. To prepare amorphous solid dispersion after screening of polymers by solvent casting method using a spray drying method. Methods. The solvent casting method was used to screen polymers, PVP/VA S-630, PVP K30, Soluplus, PEG 4000, HPMC AS, and HPMC HP55, in 1 : 1 and 2 : 3 ratios and followed by spray drying after polymer selection. Results. The dissolution performance of the formulation improved with time. The optimum feed rate and feed concentration were found to have an impact on the flow properties and particle size of spray-dried formulations, and they were selected as independent variables in a 32 full factorial statistical design. The ANOVA and regression analysis suggest that the developed regression model has a significant overall fit to the data and can explain a substantial proportion of the variability in the dissolution at 10 minutes. The optimized batch was selected based on the decisive factors of minimum and maximum values of response variables. Overall, the optimized batch demonstrated improved characteristics in terms of percentage yield (32.81%), dissolution at 10 min (49.70%), and angle of repose at 31.42°. Conclusion. This study provides valuable insights into optimizing formulation strategies for preserving the amorphous state of drugs and contributes to the development of stable pharmaceutical formulations.
Introduction
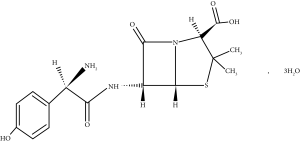
Amoxicillin trihydrate, which is chemically (2S,5R,6R)-6-[[(2R)-2-Amino-2-(4-hydroxyphenyl) acetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptanes-2-carboxylic acid trihydrate, Figure 1, is an analog of ampicillin, semisynthetic, broad-spectrum, beta-lactam antibiotic with bacteriolytic and bactericidal activity against many Gram-positive and Gram-negative microorganisms. Amoxicillin trihydrate is widely used in the treatment of genitourinary tract infections; otitis media, nose, and throat infections; upper and lower respiratory tract infections; helicobacter pylori infections; and pharyngitis, tonsillitis, and urogenital tract infections [1]. It binds to the penicillin-binding protein (PBPs) and inhibits the final step of transpeptidation in peptidoglycan synthesis and the synthesis of the bacterial cell wall.
It is generally known that antibiotic compounds in powder form are not suitable for formulation purposes, as these powders generally perform poorly when it comes to flowability, which leads to difficulties in the production of final dosage forms such as tablets. Tablets comprise the most widely used dosage form due to their low cost of manufacturing as compared to other formulations, accurate dosing, and patient convenience. Direct compression and wet or dry granulation are two methods for making tablets [2, 3].
Direct compression is simple, improves productivity, and is a cost-effective method of tablet manufacturing as compared to wet or dry granulation. Direct compression eliminates many steps (like granulation, drying, etc.) of the dry granulation and wet granulation techniques [4, 5]. However, it requires excellent flowability and compressibility, but contrarily, amoxicillin trihydrate has poor flowability and compressibility, which leads to its characteristics and direct compression is very difficult and creates challenges in the production of final dosage forms such as tablets [6]. Consistent final product quality requires precise dosing, which is challenging to achieve in the case of poor flowability. To address these problems, it requires improvements in flowability and compressibility that facilitate tablet manufacturing by direct compression.
“Solid dispersions as a technique of increasing dissolution and oral bioavailability for weakly water-soluble medicines,” Sekiguchi and Obi reported in 1961. All drugs are dispersed within a single solid carrier matrix in solid dispersion. Solid dispersion is classified based on the molecular arrangement of the drug and carrier [7]. Either the drug is a separate crystalline or amorphous particle suspended in the carrier, or the drug and carrier coexist as a single amorphous phase. Solid dispersion can be prepared using several different techniques, such as solvent evaporation, hot melt extrusion, rotary evaporation, and spray drying. [8].
In recent decades, spray drying has emerged as a prominent processing technology for developing amorphous solid dispersions of drugs. It is a one-step process that can convert suspension or solution into dry powder. In this process, the API and excipient are dissolved in a common volatile solvent and atomized into a stream of hot gas. As the solvent evaporates from the droplet, the solutes rapidly solidify, trapping the API in an amorphous state within the carrier matrix. Drugs are often in an amorphous state in solid dispersions prepared by spray drying; therefore, the solubility and the dissolution rate are significantly increased [9].
Spray drying is the most suitable technique for controlling the production and process variables of powder, such as particle size, shape, and density. It is the most important characteristic of flow property and compressibility [10]. Solid dispersion products prepared by spray drying are available commercially, such as Insweek® and Intullation®.
Based on this consideration, the objective of this research was to formulate an amorphous solid dispersion of amoxicillin trihydrate by a spray drying technique for dissolution enhancement in comparison with existing formulations and an improvement in compressibility and flow properties of amoxicillin trihydrate. This research is motivated by the pressing need to enhance the solubility and overall bioavailability of amoxicillin trihydrate. The novelty lies in the systematic exploration of various polymers, including PVP/VA S-630, PVP K30, Soluplus, PEG 4000, HPMC AS, and HPMC HP55, through solvent casting and subsequent optimization via a 32 full factorial statistical design. The formulation strategies presented herein aim to contribute to the development of stable pharmaceutical formulations with improved characteristics. While the findings contribute valuable insights into formulating strategies for preserving the amorphous state of drugs, it is crucial to acknowledge the limitations of the study. The study primarily focuses on the impact of feed rate and feed concentration on the characters like flow properties and dissolution rate of spray-dried formulations. While this investigation provides significant insights, other formulation parameters and external factors may influence the overall performance of the amorphous solid dispersion.
Download the full article as PDF here A Retrospective Analysis of Polymer Selection Using Solvent Casting
or read it here
Materials
Amoxicillin trihydrate was provided as a gift sample by Bharat Parenterals Ltd. PVP/VA S-630 (Plasdone S-630) and HPMC AS were provided as gift samples by Ashland Global. Soluplus® and Poloxamer 407 were purchased from BASF. PVP k30, PEG 4000, DCM, acetone, and methanol were purchased from Molychem Chemicals. HPMC HP55 was purchased from Hipax Ester Pvt. Ltd., Maharashtra. Kyron T314 was purchased from Doshion Polymers Solution Pvt. Ltd. Microcrystalline cellulose (MCC) was purchased from RanQ Remedies Pvt. Ltd. Aerosil was purchased from Evonik. IPA was purchased from Lucemill Ltd
Chetan Borkhataria, Hardik Chauhan, Bhargavi Mistry, Manisha Kalaria, Rachana Katbamana and Kalpesh Patel, A Retrospective Analysis of Polymer Selection Using Solvent Casting: Formulation and DoE Optimization of the Amorphous Solid Dispersion of Amoxicillin Trihydrate by a Spray Drying Method, Received 30 January 2024; Revised 14 March 2024; Accepted 25 March 2024; Published 8 April 2024, Hindawi, International Journal of Chemical Engineering, Volume 2024, Article ID 8734695, 13 pages, https://doi.org/10.1155/2024/8734695
See our next webinar:
“Rethinking the development of controlled release formulations and manufacturing processes”
Date: 30th of April, Time: 3:00 pm (Amsterdam, Berlin)


