Reversible protein complexes as a promising avenue for the development of high concentration formulations of biologics
Abstract
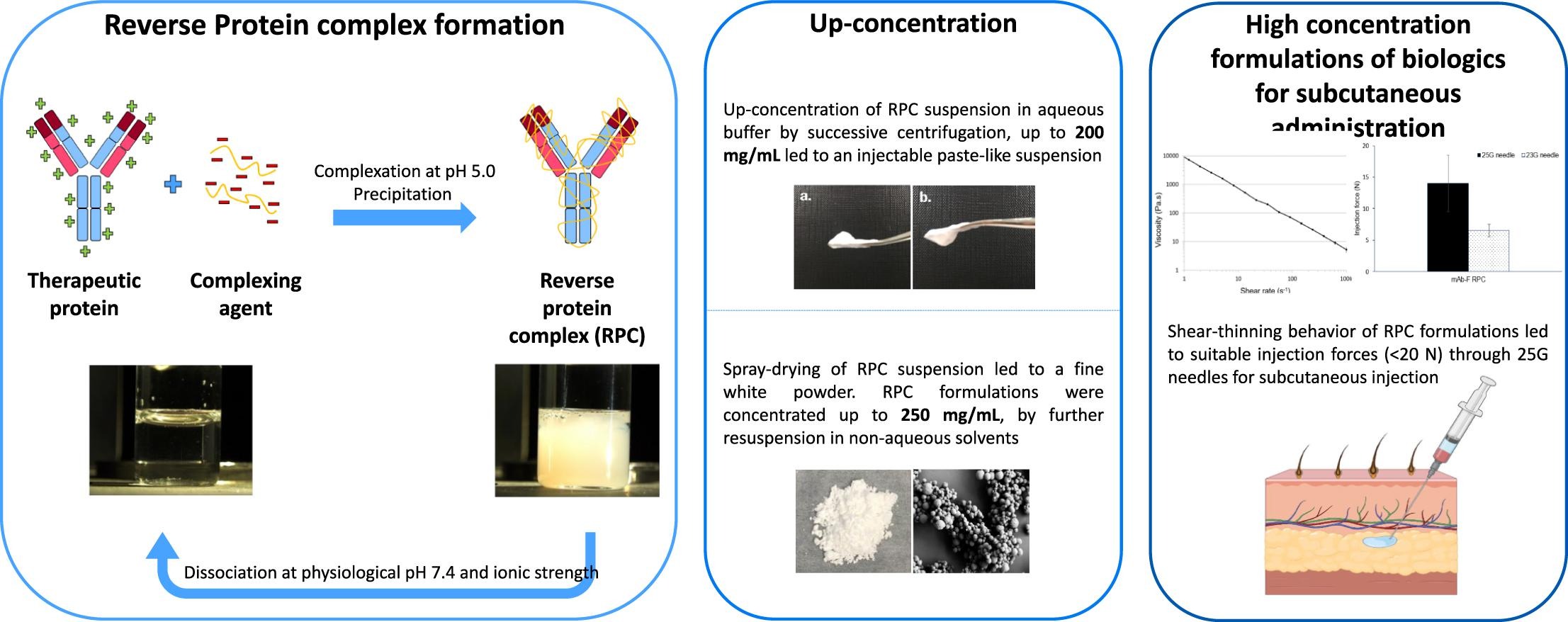
High concentration formulations have become an important pre-requisite in the development of biological drugs, particularly in the case of subcutaneous administration where limited injection volume negatively affects the administered dose. In this study, we propose to develop high concentration formulations of biologics using a reversible protein-polyelectrolyte complex (RPC) approach. First, the versatility of RPC was assessed using different complexing agents and formats of therapeutic proteins, to define the optimal conditions for complexation and dissociation of the complex. The stability of the protein was investigated before and after complexation, as well as upon a 4-week storage period at various temperatures. Subsequently, two approaches were selected to develop high concentration RPC formulations: first, using up-concentrated RPC suspensions in aqueous buffers, and second, by generating spray-dried RPC and further resuspension in non-aqueous solvents. Results showed that the RPC concept is applicable to a wide range of therapeutic protein formats and the complexation-dissociation process did not affect the stability of the proteins. High concentration formulations up to 200 mg/mL could be achieved by up-concentrating RPC suspensions in aqueous buffers and RPC suspensions in non-aqueous solvents were concentrated up to 250 mg/mL. Although optimization is needed, our data suggests that RPC may be a promising avenue to achieve high concentration formulations of biologics for subcutaneous administration.
Introduction
Over the last couple of decades, biological drugs have been gradually taking over small molecules in terms of the number of FDA drug approvals. While new biological entities represented 10 % of the total approved new molecular entities from 1993 to 1999, they increased to 17 % from 2000 to 2009 and to 24 % from 2010 to 2019. This trend is persisting in the early 2020 s with 25 % and 28 % of approvals in 2020 and 2021, respectively, and up to 44 % in 2022, including the approvals of the 100th antibody drug and novel biologic formats (Mullard, 2023). This can be attributed to the good tolerability of biologics, owing to their biological origin over the chemically synthetized small molecules, but also to their higher selectivity, particularly the last generations of biologics, exhibiting specific or even bispecific targeting (Shirley, 2022), while being increasingly more potent, thereby improving safety and efficacy.
Biologic drugs are now standard of care and have improved patients’ lives in various indications, including oncology, immunology, ophthalmology and many rare diseases. However, there are some key technical limitations to enable their use at a larger scale, and one of them is the route of administration. Due to the inherent physico-chemical properties of biologics, oral administration of therapeutic proteins proved to be ineffective due to the degradation by acidic stomach pH or digestive enzymes, and to a limited permeation through the gastrointestinal tract. Although promising work has been pioneered to improve oral administration of biologics (Truong-Le et al., 2015), no drug product has made it to the market so far. Furthermore, several alternative routes of administration have been investigated (Morales et al., 2017), with only a few successful attempts in the field of inhalation (Greene and Finch, 2021). Therefore, biologics are predominantly commercialized as solutions for parenteral administration. To ensure protein stability during shipment/handling and over long-term storage, biologics are generally formulated in aqueous liquid/lyophilized formulations (Basle et al., 2019). Moreover, biological products are most frequently stored at 5 ± 3 °C, resulting in a complicated and costly supply chain, and decreasing the accessibility to wider population, especially in the developing countries (Ibrahim and Araujo, 2021).
Generally, biologic drugs are administered via intravenous (IV) injection/infusion to allow a high bioavailability, which is difficult to achieve using other routes of administration. Lately, a clear trend towards subcutaneous (SC) formulation development has been observed in certain therapeutic areas. The SC route of administration presents many advantages compared to intravenous administration, which has a lower patient compliance and exhibits a significant burden on the time and resources of patients, healthcare professionals and the healthcare system at large (Bittner et al., 2018, Jonaitis et al., 2021). Looking even further, the development of auto-injectors for SC administration has allowed patients to self-medicate at home (Bemt et al., 2019), improving compliance and reducing the overall cost for society and healthcare systems.
One of the main limitations encountered with SC administration is the relatively high dose in limited volume (≤2 mL) to avoid pain or adverse events at the injection site (Badkar et al., 2021, Mathaes et al., 2016, Sequeira et al., 2019). Recently, different formulation or administration device strategies to increase the SC injection volume have been developed. For instance, the co-formulation of protein therapeutics with hyaluronidase (rHuPH20) to loosen the interstitial space by hydrolyzing hyaluronan in the SC extra cellular matrix upon injection, allowed increasing the injection volume up to 20 mL when administered using an infusion pump (Frost, 2007, Locke et al., 2019, Shpilberg and Jackisch, 2013). Another formulation-based approach to overcome the injection volume limitations is to increase the concentration of the biological drug in the formulation to allow the delivery of a higher dose via a single injection. However, such high concentration formulations (>100 mg/mL) often lead to reduced processibility, stability and excessive viscosity, preventing their administration via injection due to high injection forces (Burckbuchler et al., 2010, Schermeyer et al., 2017, Watt et al., 2019). As such, the development of innovative high concentration formulations of biologics is of high interest to improve the SC delivery of therapeutic proteins.
In this study, we present a way of developing such formulations using a reversible protein-polyelectrolyte complexation concept to tackle the aforementioned formulation challenges. Reversible protein complexes (RPC), also referred to as hydrophobic ion pairing (Ristroph and Prud’homme, 2019), protein-polyelectrolyte complexes (Gao et al., 2019) or polyion complexes (Insua et al., 2016), have already been widely reported in the literature and were first introduced by Morawetz and Hughes in 1952 as a purification method (Morawetz and Hughes, 1952). Their use has since evolved towards other applications, including drug delivery systems for biologics.
The RPC concept consists of mixing oppositely charged molecules under controlled physico-chemical conditions (e.g. pH, ionic strength and mole-charge ratio) leading to the formation of a complex via electrostatic interactions. Since the charges are neutralized and masked, the macromolecular complex is not soluble anymore and precipitates as whitish particles resulting in a suspension. Importantly, this complexation is reversible by decreasing the electrostatic interactions, which are sensitive to the surrounding pH and ionic strength. Increasing the ionic strength causes an electrostatic shielding between the oppositely charged molecules, whereas changing the pH results in reducing the charge distribution on the molecules, leading to a decrease in the electrostatic protein-polyelectrolyte interactions and consequent dissociation of the two molecules.
This concept has been assessed with biological molecules, including enzymes (Takahashi et al., 2000), peptides (Lu et al., 2018) or proteins (Patel et al., 2014). Originally described as a purification method, it has recently been proposed as a drug delivery strategy. Many studies have reported its use in combination with drug carriers in targeted therapies to enhance bioavailability. Herein, the RPC concept was used to increase the hydrophobicity of the therapeutic molecules and increase their incorporation efficiency in already established drug delivery systems, such as micelles (Gao et al., 2019), nanoparticles (Patel et al., 2014), liposomes (Xu et al., 2017) and self-emulsifying drug delivery systems (Griesser et al., 2017). Its use as a formulation strategy to achieve high concentration formulations of biologics has also been reported (Kurinomaru et al., 2014, Kurinomaru and Shiraki, 2017, Tsumura et al., 2022). Our work uniquely highlights the broad versatility of the RPC concept. Once the best complexing agent was selected using diverse and state of the art therapeutic protein formats, the robustness of the RPC formulation was tested. The physical, chemical and performance stabilities of the RPC formulations were evaluated upon a 4-week storage period at different temperature conditions. Then, and finally meeting the objective of this work, high concentration formulations of biologics using RPC were explored using two different techniques, namely direct up-concentration as aqueous suspensions, or dried RPC particles resuspension in a non-aqueous solvent.
Read more here
Materials
All therapeutic proteins were manufactured and purified by F. Hoffmann-La Roche AG (Basel, Switzerland). Histidine-HCl and L-histidine base were obtained from Ajinomoto (Osaka, Japan), citric acid and trisodium citrate from Merck (Darmstadt, Germany), polysorbate 20 (PS20) from Croda (East Yorkshire, UK), poloxamer 188 (Px188) from BASF (Ludwigshafen, Germany) and sucrose from Pfanstiehl (Zug, Switzerland).
Naoual Dahmana, Pierre-Louis Destruel, Samantha Facchetti, Vanessa Braun, Vanessa Lebouc, Zana Marin, Sulabh Patel, Gregoire Schwach, Reversible protein complexes as a promising avenue for the development of high concentration formulations of biologics, International Journal of Pharmaceutics, Volume 648, 2023, 123616, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.123616.

