Determination of Gastric Water Emptying in Fasted and Fed State Conditions Using a Compression-Coated Tablet and Salivary Caffeine Kinetics
Abstract
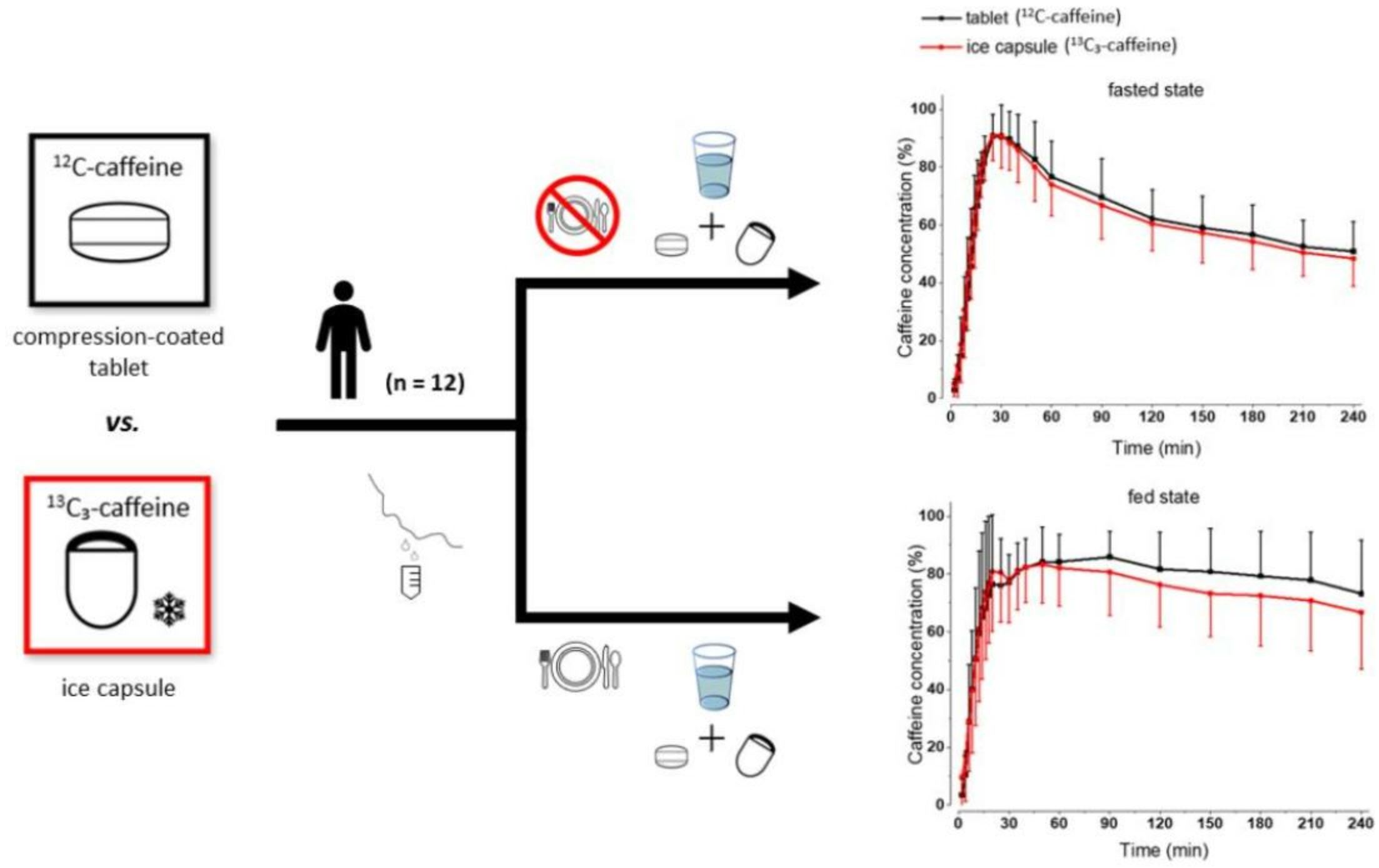
Because of the importance of gastric emptying for pharmacokinetics, numerous methods have been developed for its determination. One of the methods is the salivary tracer technique, which utilizes an ice capsule containing caffeine as a salivary tracer. Despite the ice capsule’s advantage in labeling ingested fluids with caffeine for subsequent salivary detection, its risk of premature melting before swallowing, and its complicated storage and preparation, limit its application, particularly in special populations (e.g., older people). For this reason, here, a compression-coated tablet was developed and validated against the ice capsule in a cross-over clinical trial. The two dosage forms were administered simultaneously to 12 volunteers in an upright position under fasted and fed state conditions. To distinguish the caffeine concentrations in saliva from each dosage form, regular type of caffeine (12C) was added to the tablet, while for the ice capsule 13C3 labelled caffeine was used. The salivary caffeine concentrations showed no statistically significant differences for the pharmacokinetic parameters tmax and AUC0→60 (p > 0.05). Thus, the new formulation is a useful tool for determining gastric emptying that can also be used in special populations.
Introduction
Oral drug delivery is the most common route of administration, because of its non-invasiveness, patient adherence and safety [1,2]. As orally administered drugs must pass through the stomach before being absorbed in the intestines, gastric emptying time is an important physiological factor influencing their absorption kinetics [3,4,5,6]. The emptying process for non-caloric liquids (i.e., water) is typically described by first-order kinetics. In young healthy adults it has been found that 240 mL of still water is emptied from the stomach within 15–45 min, in both fasted and fed conditions. Unaltered emptying under postprandial conditions is due to the phenomenon of Magenstrasse, which describes a “gastric route” formed inside the fed stomach that allows the rapid emptying of water into the small intestine, and consequently of the dissolved or suspended drug substance [7,8,9]. On the contrary, less is known for the gastric emptying of liquids in geriatric patients and older people [10].
Most commonly, gastric emptying is determined by the performance of imaging studies, such as an MRI, which has a high cost and requires specialized equipment, and scintigraphy with the additional risk of radiation exposure. Pharmacokinetic markers are among the well-established methods; however, they may require frequent blood sampling [11,12]. For older people and geriatric patients, more simplified methods are needed, as they can allow for an application of the method to a wider range of populations. For instance, the salivary tracer technique, previously developed by Sager et al., is a non-invasive method for the determination of gastric water emptying, using an ice capsule containing a caffeine solution, since caffeine is used as a salivary marker. The volunteer can effortlessly collect saliva samples himself, allowing repeated sample collection for estimation of gastric emptying time either for physiological evaluations or during pharmacokinetic studies [13].
However, the ice capsule exhibits some limitations, which hinder its broad application, such as its size (15 mm diameter) which makes it difficult to swallow, especially for patients who have swallowing difficulties [14,15]. Moreover, its room temperature instability can result in rapid melting before intake, or caffeine contamination of the oral cavity, if not swallowed immediately. It is also difficult to use it in a broad range of settings (e.g., geriatric wards and hospitals), as the ice capsules must be frozen during production and transport. All of these factors can subsequently lead to the failure of this method.
The goal of this study was to develop an alternative vehicle for rapid delivery of caffeine into the stomach content. The new vehicle should present the following properties: (1) It releases caffeine just as quickly as the ice capsule, and therefore leads to emptying of the caffeine to the duodenum with the ingested water. (2) It remains stable at room temperature and in the oral cavity avoiding salivary contamination with caffeine while intake. (3) It has a small and convenient size. (4) It is easier to produce, store and transport. As with ice capsules, it is important to choose a preparation method that ensures that caffeine is only present within the vehicle and to prevent caffeine migration to the surface during the production process and during storage. The new vehicle was designed as a fast disintegrating tablet; however, a mechanism to avoid contamination of the oral cavity with caffeine during administration was mandatory. Due to the typically small volumes of saliva in the oral cavity, even the smallest amounts of caffeine would cause oral contamination. Therefore, a film-coated tablet was ruled out as an option, as there would be the risk of the caffeine from the tablet cores dissolving during the coating process, and thus getting into the coating [16]. For this reason, a compression-coated tablet was developed to optimize the application of the salivary tracer technique. The compression-coated tablet consisted of an inner core containing caffeine and an outer layer to protect the oral cavity from caffeine contamination.
The tablet was validated against the ice capsule, in an open cross-over clinical study of two study arms, under fasted and fed conditions in accordance with the recommendations for bioavailability/bioequivalence (BA/BE) studies [17,18]. In order to determine whether the tablet could detect gastric water emptying, the two dosage forms were given simultaneously to volunteers in an upright position. To distinguish the salivary caffeine concentrations natural 12C-caffeine was added to the tablet, and stable isotope labelled 13C3-caffeine was added to the ice capsule. The use of a stable isotope to monitor the release and absorption of caffeine measured by caffeine concentrations in saliva has already been applied to a recent study of in vivo oral dosage form disintegration [19], since 12C- and 13C3-caffeine have similar pharmacokinetic profiles [20].
Download the full article as PDF here Determination of Gastric Water Emptying in Fasted and Fed State Conditions Using a Compression-Coated Tablet and Salivary Caffeine Kinetics
or read it here
Materials
The composition of the compression-coated tablets and the respective quantities of the ingredients used are provided in Table 1 and Table 2. 12C-caffeine, saccharin sodium, Methocel E4M® (hydroxypropyl methylcellulose) and black iron oxide were purchased from Caesar & Loretz GmbH (Hilden, Germany). Vivasol® (croscarmellose sodium) and Prosolv® (silicified microcrystalline cellulose) was obtained from JRS Pharma GmbH & Co. KG (Barsbüttel, Germany). Aerosil® (amorphous colloidal silicon dioxide), Avicel® (microcrystalline cellulose) and magnesium stearate were kindly gifted from F. Hoffmann-La Roche AG (Basel, Switzerland). 13C3-caffeine was purchased from Sigma-Aldrich Chemie GmbH (Schnelldorf, Germany). Aluminium bags used for packaging the tablets were purchased from Ströbel GmbH (Langenzenn, Germany). All solvents used for HPLC, i.e., water and methanol were of analytical grade, and all solvents used for LC-MS were of LC-MS grade.
Tzakri, T.; Rehenbrock, L.; Senekowitsch, S.; Rump, A.; Schick, P.; Krause, J.; Kromrey, M.-L.; Grimm, M.; Weitschies, W. Determination of Gastric Water Emptying in Fasted and Fed State Conditions Using a Compression-Coated Tablet and Salivary Caffeine Kinetics. Pharmaceutics 2023, 15, 2584. https://doi.org/10.3390/pharmaceutics15112584
Read more on Magnesium Stearate as a pharmaceutical excipient here:


