Development of Simvastatin-Loaded Particles Using Spray Drying Method for Ex Tempore Preparation of Cartridges for 2D Printing Technology
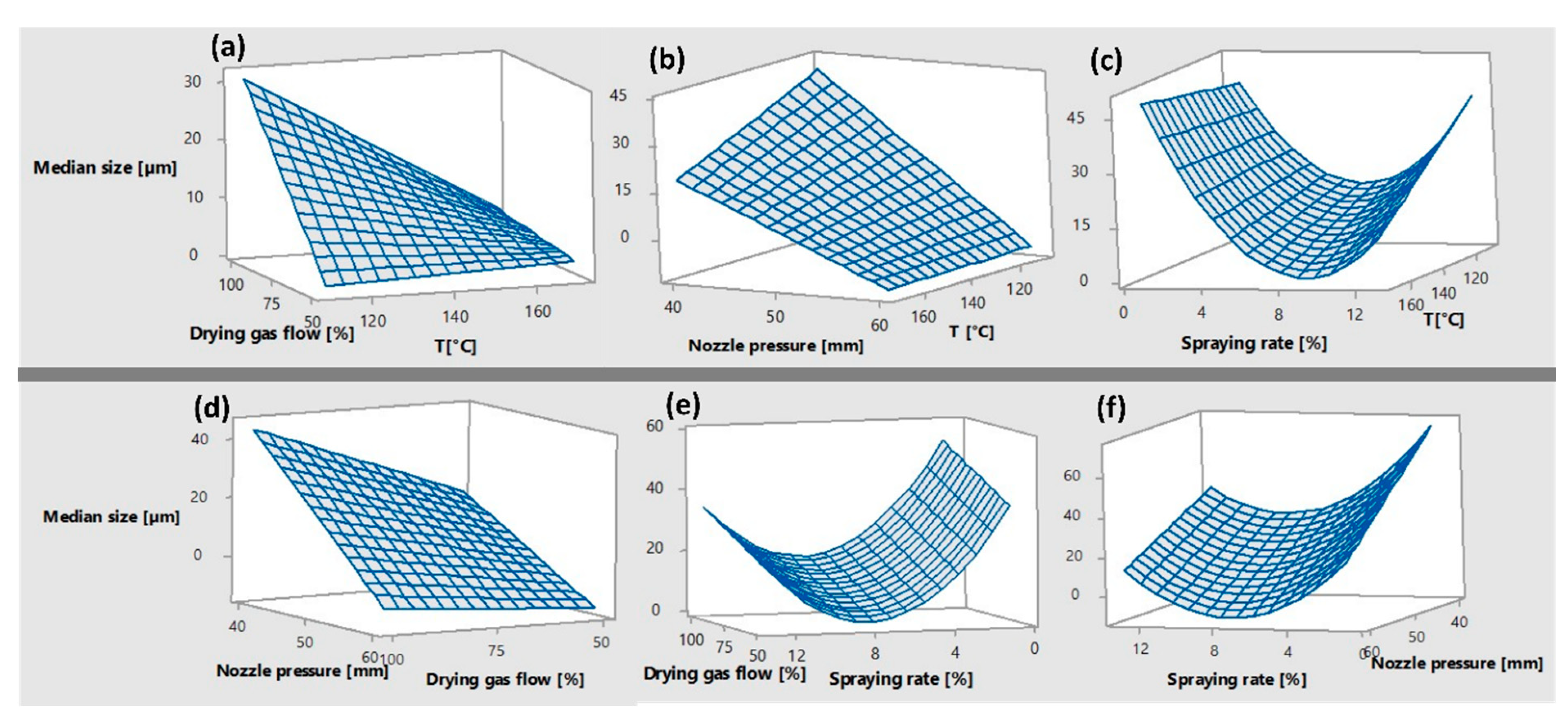
In this work, a spray drying method was developed to produce drug/polymer (simvastatin/polycaprolactone) microparticles that have the potential to be used as a pre-formulation for ex tempore preparation of 2D printing cartridges. An experimental model was designed with the process parameters set to predict the smallest particle size required for successful 2D printing. Three different types of particles (lactose, nanocellulose/lactose, calcium silicate) were produced, and the average size of the dry particles varied depending on the sampling location (cyclone, collection vessel). The encapsulation efficiency of simvastatin was highest with nanocellulose/lactose from the collection vessel. The one-month stability of simvastatin in the particles showed low content, but the addition of ascorbic acid as an antioxidant increased the chemical stability of the drug.
Interestingly, the addition of antioxidants decreased the stability of simvastatin in the calcium silicate particles from the collection vessel. Dispersion of the particles in three different propylene glycol and water mixtures (10/90, 50/50, and 90/10% (v/v)), representing a printable ink medium with three different viscosity and surface tension properties, showed that nanocellulose/lactose was the most suitable antiadhesive in terms of dispersed particle size (˂1 µm). After one month of storage, the dispersed particles remained in the same size range without undesirable particle agglomeration.
Download the full article as PDF here Development of Simvastatin-Loaded Particles Using Spray Drying Method for Ex Tempore Preparation of Cartridges for 2D Printing Technology
or read it here
Materials
Simvastatin (SIM) was kindly donated by Krka d.d. (Novo Mesto, Slovenia); polycaprolactone (PCL) of 14,000 daltons, polysorbate (Tween ® 20), propylene glycol, and ascorbic acid (AA) were purchased from Sigma Aldrich, St. Louis, MO, USA. Lactose Mesh 200 donated by Lek d.d. (Slovenia), Nanocelullose (NCC, Cellu Force, Montreal, QC, Canada), and Florite R calcium silicate (CaSi, Tomita Pharmaceutical, Tokushima, Japan) were used as antiadhesives in spray drying experiments. Chloroform (Merck KGaA, Darmstadt, Germany) and purified water were used as O/W emulsion phases. The solvents used for U(H)PLC analysis were of HPLC grade. All other reagents used were of analytical grade. Water for UPLC analysis was purified using a Milli-Q system with a 0.22 Millipak 40 filter (Millipore, Cork, Ireland).
Sterle Zorec, B.; Dreu, R. Development of Simvastatin-Loaded Particles Using Spray Drying Method for Ex Tempore Preparation of Cartridges for 2D Printing Technology. Pharmaceutics 2023, 15, 2221. https://doi.org/10.3390/pharmaceutics15092221
Read also more on Introduction to Stearic Acid as a pharmaceutical Excipient here:


