Modification of microenvironmental pH of nanoparticles for enhanced solubility and oral bioavailability of poorly water-soluble celecoxib
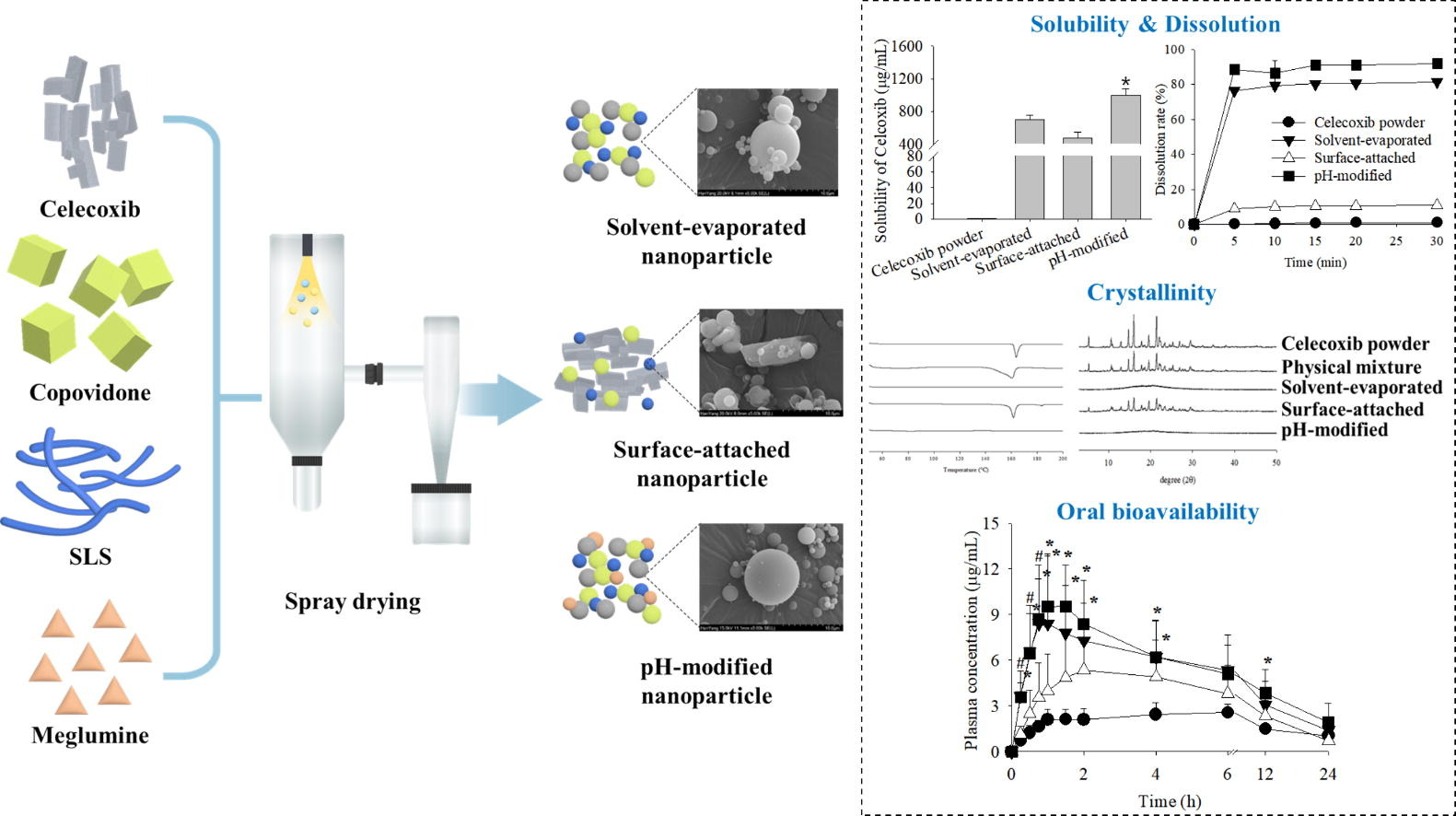
This study aimed to develop a novel pH-modified nanoparticle with improved solubility and oral bioavailability of poorly water-soluble celecoxib by modifying the microenvironmental pH. After assessing the impact of hydrophilic polymers, surfactants and alkaline pH modifiers on the drug solubility, copovidone, sodium lauryl sulfate (SLS) and meglumine were chosen. The optimal formulation of solvent-evaporated, surface-attached and pH-modified nanoparticles composed of celecoxib/copovidone/SLS/meglumine at weight ratios of 1:1:0.2:0, 1:0.375:1.125:0 and 1:1:1:0.2:0.02, respectively, were manufactured using spray drying technique.
Highlights
- pH-modified nanoparticles were prepared with copovidone, SLS and meglumine.
- The microenvironment pH of nanoparticles was modified by adding meglumine.
- pH-modified nanoparticles showed conversion from crystalline to amorphous drug.
- pH-modified nanoparticles showed the highest solubility and oral bioavailability.
Their physicochemical characteristics, solubility, dissolution and pharmacokinetics in rats were evaluated compared to the celecoxib powder. The solvent-evaporated and pH-modified nanoparticles converted a crystalline to an amorphous drug, resulting in a spherical shape with a reduced particle size compared to celecoxib powder. However, the surface-attached nanoparticles with insignificant particle size exhibited the unchangeable crystalline drug. All of them gave significantly higher solubility, dissolution, and oral bioavailability than celecoxib powder.
Among them, the pH-modified nanoparticles demonstrated the most significant improvement in solubility (approximately 1600-fold) and oral bioavailability (approximately 4-fold) compared to the drug powder owing to the alkaline microenvironment formation effect of meglumine and the conversion to the amorphous drug. Thus, the pH-modified nanoparticle system would be a promising strategy for improving the solubility and oral bioavailability of poorly water-soluble and weakly acidic celecoxib.
Read more here
Materials
Celecoxib, SLS, meglumine, hydroxypropyl-β-cyclodextrin (HP-β-CD) and calcium silicate were obtained from Hanmi Pharm. Co. (Suwon, South Korea). Gelatin, sorbitan monooleate (Span 20), polysorbate (Tween 20), potassium carbonate, sodium bicarbonate, calcium hydroxide, precipitated calcium carbonate and magnesium carbonate were purchased from Daejung Chem. Co. (Siheung, South Korea). Hydroxypropylmethylcellulose (HPMC 2910 P606), hydroxypropylmethylcellulose 4000 (HPMC 4000) and Klucel® from Ashland.
Mi Ran Woo, Young-Woo Bak, Seunghyun Cheon, Jung Suk Kim, Sang Hun Ji, Seonghyeon Park, Sanghyun Woo, Jong Oh Kim, Sung Giu Jin, Han-Gon Choi, Modification of microenvironmental pH of nanoparticles for enhanced solubility and oral bioavailability of poorly water-soluble celecoxib, International Journal of Pharmaceutics, 2024, 124179, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.124179.
Vitafoods Geneva 2024 – with a focus on excipients


