A step forward on the in vitro and in vivo assessment of a novel nanomedicine against melanoma
Abstract
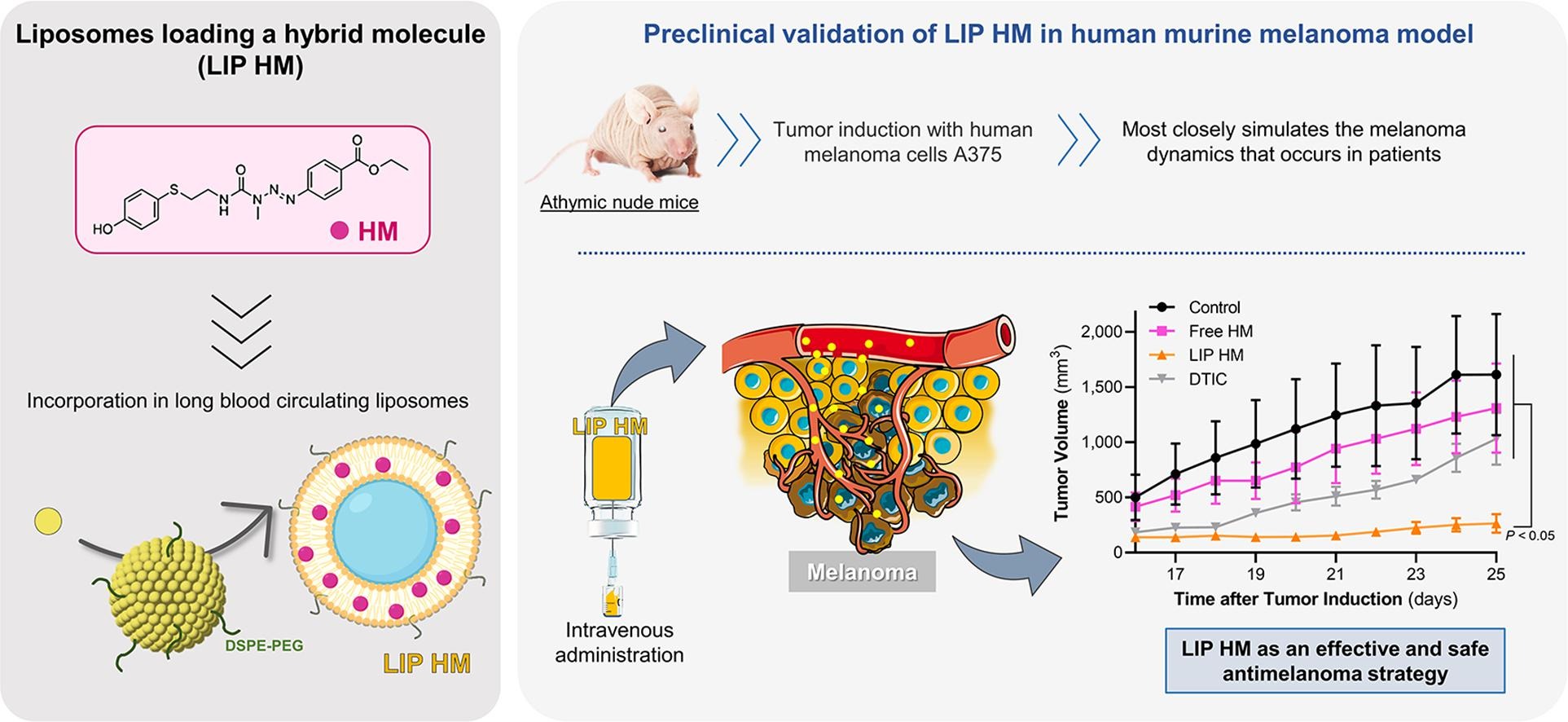
Melanoma is the most aggressive form of skin cancer, with increasing incidence and mortality rates. To overcome current treatment limitations, a hybrid molecule (HM) combining a triazene and a sulfur L-tyrosine analogue, was recently synthesized, incorporated in long blood circulating liposomes (LIP HM) and validated in an immunocompetent melanoma model. The present work constitutes a step forward in the therapeutic assessment of HM formulations. Here, human melanoma cells, A375 and MNT-1, were used and dacarbazine (DTIC), a triazene drug clinically available as first-line treatment for melanoma, constituted the positive control. In cell cycle analysis, A375 cells, after 24-h incubation with HM (60 μM) and DTIC (70 μM), resulted in a 1.2 fold increase (related to control) in the percentage of cells in G0/G1 phase. The therapeutic activity was evaluated in a human murine melanoma model (subcutaneously injected with A375 cells) to most closely resemble the human pathology. Animals treated with LIP HM exhibited the highest antimelanoma effect resulting in a 6-, 5- and 4-fold reduction on tumor volume compared to negative control, Free HM and DTIC groups, respectively. No toxic side effects were detected. Overall, these results constitute another step forward in the validation of the antimelanoma activity of LIP HM, using a murine model that more accurately simulates the pathology that occurs in human patients.
Highlights
- HM, an hybrid molecule combining a triazene and a tyrosine analogue, was validated in vitro and in vivo against melanoma.
- In vitro, HM displayed higher antiproliferative properties than dacarbazine towards commercial and patient-derived melanoma cells.
- In vivo, HM antimelanoma activity, was potentiated after incorporation in long blood circulating liposomes.
The therapeutic effect of HM liposomes was clearly superior to free HM and dacarbazine.
Introduction
Melanoma is the most aggressive and fatal form of skin cancer, arising from the malignant conversion of melanocytes, the cells involved in melanin production (Pinho et al., 2019b). If untreated, the disease progresses and metastasizes to lymph nodes and distant organs, considerably increasing mortality (Massand and Neves, 2021). The development of this malignancy is attributed to the interaction of different factors, namely genetic (e.g. inherited mutations, polymorphisms, family history) and environmental (e.g. ultraviolet radiation, successive sunburns, use of indoor tanning, dietary factors) (Dzwierzynski, 2021, Pinho et al., 2021b). According to the International Agency for Research on Cancer, it is expected a continuous and alarming increase in the worldwide incidence (325 vs 425 thousand people at years 2020 vs 2040, respectively) and mortality (57 vs 85 thousand people at years 2020 vs 2040, respectively) of malignant melanoma (GLOBOCAN., 2022). These facts, together with the lack of effective and safe treatments, prompt the development of novel therapeutic alternatives to improve clinical outcomes.
Recently, compounds with enhanced anticancer activity have been synthesized through molecular hybridization (Alsayari et al., 2022, Aly et al., 2020, Granada et al., 2021, Sun et al., 2022). This approach combines two or more pharmacophores in one single chemical entity that acts via different mechanisms of action. These compounds present several advantages, such as higher affinity, selectivity and efficacy, as well as lower potential to elicit undesired side effects and ability to reduce resistances compared to monotherapy (Claudio Viegas-Junior et al., 2007, Francisco et al., 2019, Szumilak et al., 2021). Applying this promising strategy, our group has synthesized a set of hybrid molecules with antimelanoma properties. For this, 4-S-cysteaminylphenol (4-S-CAP), a ʟ-tyrosine sulfur analogue with specific melanocytotoxicity, was combined with a triazene moiety, presenting DNA alkylating effects (Granada et al., 2021).
In particular, one of the synthesized hybrid molecules, here after designated as HM, showed the best antimelanoma properties in vitro (Granada et al., 2021). Indeed, a screening against several cancer cell lines revealed a higher antiproliferative activity of HM compared to temozolomide (TMZ), a triazene drug used in the clinic against melanoma brain metastasis (Pinho et al., 2023). This HM cytotoxic effect was associated to the halt of cell cycle and apoptosis of melanoma cells, as demonstrated in the murine melanoma cell line B16-F10. Further, to enhance in vivo therapeutic efficacy, HM was associated to long blood circulating liposomes (LIP HM). In preclinical studies using a B16-F10 syngeneic subcutaneous melanoma model, a notable reduction on tumor progression for mice treated with LIP HM was attained. Also, in a B16-F10 metastatic melanoma model, LIP HM reduced the number of lung metastases, in a higher extent as compared with Free HM (Pinho et al., 2023).
Capitalizing on the encouraging data for LIP HM (Pinho et al., 2023), in the present work we aimed to further validate this therapeutic strategy using human melanoma cell line models. HM mechanisms of actions, namely cell cycle and caspase 3/7 activity, were investigated using two human melanoma cell lines, A375 and MNT-1. Data was compared with dacarbazine (DTIC), a triazene derivative used as first-line chemotherapy for malignant melanoma (Marchesi et al., 2007, Skibba et al., 1970). For the in vivo therapeutic evaluation, human melanoma cells, A375, were injected subcutaneously in immunocompromised mice and four experimental groups were established: negative Control, positive control (DTIC), Free HM and LIP HM. This xenograft model provides important information regarding human disease biology and progression that, in turn, allows a more reliable prediction of patients’ therapeutic response.
Download the full article here A step forward on the in vitro and in vivo assessment of a novel nanomedicine against melanoma
or read it here
Materials
Dimethyl sulfoxide (DMSO), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and dacarbazine (DTIC) were obtained from Sigma-Aldrich (St Louis, MO, USA). Cell culture media and antibiotics were purchased from Invitrogen (Life Technologies Corporation, NY, USA). Reagents for cell proliferation assays were acquired from Promega (WI, USA). The pure phospholipids egg phosphatidyl choline (PC) and distearoyl phosphatidyl ethanolamine covalently linked to polyethylene glycol 2000 (DSPE-PEG), used for the preparation of liposomal nanoformulations, were purchased from Avanti Polar Lipids (AL, USA). All the remaining chemicals used were of analytical grade. Deionized water (Milli-Q system; Millipore, Tokyo) was used in all experiments.
Jacinta O. Pinho, Mariana Matias, Ana Godinho-Santos, Joana D. Amaral, Eduarda Mendes, Maria Jesus Perry, Ana Paula Francisco, Cecília M.P. Rodrigues, M. Manuela Gaspar, A step forward on the in vitro and in vivo assessment of a novel nanomedicine against melanoma, International Journal of Pharmaceutics, Volume 640, 2023, 123011, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2023.123011.
Visit our new Webinar:
Solving capping challenges using mannitol as an excipient model
Get more information & register here:


