Exploiting synergistic effects of brittle and plastic excipients in directly compressible formulations of sitagliptin phosphate and sitagliptin hydrochloride
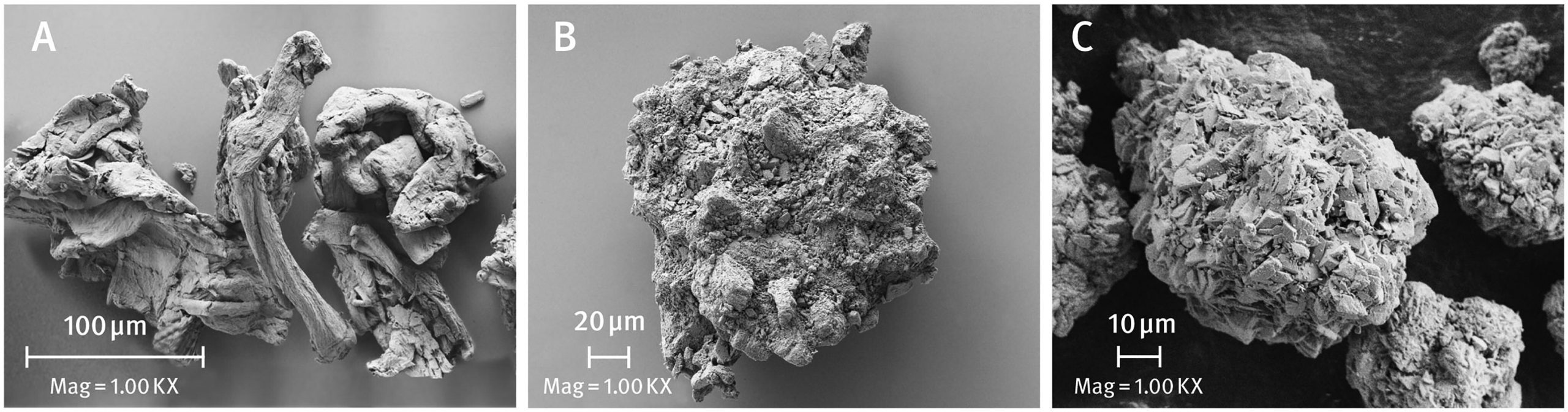
Direct compression (DC) is the simplest and most economical way to produce pharmaceutical tablets. Ideally, it consists of only two steps: dry blending of a drug substance(s) with excipients followed by compressing the powder mixture into tablets. In this study, immediate-release film-coated tablets containing either Sitagliptin phosphate or Sitagliptin hydrochloride were developed using DC technique. After establishing the optimum ratio of ductile and brittle excipients, five formulations were compressed into tablets using a rotary press and finally film coated. Both powders and tablets were examined by standard pharmacopoeial methods. It has been shown that the simultaneous use of excipients with different physical properties, i.e. ductile microcrystalline cellulose and brittle anhydrous dibasic calcium phosphate, produces a synergistic effect, allowing preparation of Sitagliptin DC tablets with good mechanical strength (tensile strength over 2 N/mm2), rapid disintegration (shorter than 2 min), and fast release of the drug substance (85% of the drug is dissolved within 15 min). It was found that the type of calcium phosphate excipient used had a large effect on the properties of the sitagliptin tablets. All formulations developed showed good chemical stability, even when stored under stress conditions (50 °C/80% RH).
Download the complete research paper as PDF: Exploiting synergistic effects of brittle and plastic excipients in directly compressible formulations of sitagliptin phosphate and sitagliptin hydrochloride
Materials
Drug substances: Sitagliptin phosphate monohydrate (Moehs BCN, S.L., Barcelona, Spain) and Sitagliptin hydrochloride monohydrate (Amino Chemicals, Marsa, Malta).
Fillers/diluents: MCC VIVAPUR® 102 from JRS Pharma (Rosenberg, Germany), DCPA DI-CAFOS® A60 and DI-CAFOS® A150 from Chemische Fabrik Budenheim KG (Budenheim, Germany).
Disintegrant: Croscarmellose sodium Ac-Di-Sol® SD-711 from FMC BioPolymer (Brussels, Belgium).
Lubricants: Magnesium stearate Ligamed® MF-2-V from Peter Greven Fett-Chemi (Venlo, The Netherlands), sodium stearyl fumarate ALUBRA® PG 100 from FMC BioPolymer (Newark, USA).
Film-coating system: AquaPolish® P pink 640.20 PVA produced by Biogrund GmbH (Huenstetten, Germany).
Reference products: Januvia® 25 mg, 50 mg, and 100 mg film-coated tablets (Merck Sharp & Dohme B.V., Haarlem, The Netherlands).
(2022) Exploiting synergistic effects of brittle and plastic excipients in directly compressible formulations of sitagliptin phosphate and sitagliptin hydrochloride, Pharmaceutical Development and Technology, DOI: 10.1080/10837450.2022.2107013

