Tableting of coated multiparticulates: Influences of punch face configurations
Abstract
The influences of the punch face design on multi-unit pellet system (MUPS) tablets were investigated. Drug-loaded pellets coated with sustained release polymer based on ethylcellulose or acrylic were compacted into MUPS tablets. Punch face designs used include standard concave, deep concave, flat-faced bevel edge and flat-faced radius edge. MUPS tablets compacted at 2 or 8 kN were characterized for their tensile strength. The extent of pellet coat damage after tableting was evaluated from drug release profiles. Biconvex tablets were weaker by 0.01–0.15 MPa, depending on the pellet type used, and had 1–17 % higher elastic recovery (p < 0.000) than flat-faced tablets. At higher compaction force, the use of the deep concave punch showed a 13–26 % lower extent of pellet coat damage, indicated by a relatively higher mean dissolution time, compared to other punch face configurations (p < 0.000). This was attributed to increased rearrangement energy of the compacted material due to the high punch concavity, which sequestered compaction stress exerted on pellet coats. Although the deep concave punch reduced the stress, the resultant tablets containing pellets coated with acrylic were weaker (p = 0.01). Overall, the punch face configuration significantly affected the quality of MUPS tablets.
Introduction
Sustained release formulations have gained popularity as a drug delivery system owing to the convenience of reduced dosing frequency and reduced incidences of side effects (Chen et al., 2017, Follonier et al., 1995, Varum et al., 2010). One approach to developing sustained release formulations is the preparation of multiparticulates (Abdul et al., 2010). A multiparticulate dosage form represents an ensemble of many small independent drug delivery units (particulates) of narrow size distribution, in the range of 150 μm to 3 mm (Rahman et al., 2009, Rajabi-Siahboomi, 2017). Pellets are often used as the particulate of choice because they are smooth surface spheroids of narrow size distribution as compared to other types of particulates such as granules or mini-tablets (Sinha et al., 2009). These physical attributes of pellets facilitate the coating of polymers that can control drug release and subsequent dispensing into unit doses.
Pellets may be filled into hard gelatin capsules or sachets, but increasingly the interest is to compact the pellets into multi-unit pellet system (MUPS) tablets (Bhad et al., 2010, Chen et al., 2017, Dreu et al., 2011, Elsergany et al., 2020a, Elsergany et al., 2020b, Thio et al., 2022). MUPS tablets are preferred over capsules due to the higher production rate of tablets, avoidance of animal-based gelatin, and ability to score the tablet with break lines to further subdivide the dosage (Chen et al., 2017). Additionally, MUPS tablets are less likely to adhere onto the esophageal lining during swallowing and are harder to tamper with, which are the major concerns with hard capsules (Fields et al., 2015). However, segregation of pellets and filler excipients can affect the drug content uniformity of MUPS tablets and requires attention (Elsergany et al., 2020a, Habib et al., 2002, Kucera et al., 2011, Osei-Yeboah et al., 2017, Tan and Hu, 2016). Despite the advantages of administering pellets as MUPS tablets (Bhad et al., 2010, Chen et al., 2017, Govender et al., 2021, Hamman et al., 2018), compaction of coated pellets remains a challenge. This is because compaction exerts considerable mechanical stress on the functional pellet coat which results in pellet coat damage (Clelik and Maganti, 1994, Bodmeier, 1997, Altaf et al., 1998, Yao et al., 1998, Chin et al., 2016, Xu et al., 2016, Elsergany et al., 2020a, Elsergany et al., 2020b, Hiew et al., 2020, Thio et al., 2022, Thio et al., 2023). Damage to the pellet coat could diminish its sustained release function or cause it to fail, particularly for highly water soluble drugs such as metformin hydrochloride (Zeeshan et al., 2009). In such a case, mitigation of compaction-induced pellet coat damage is required.
Factors in the formulation and process variables to mitigate compaction-induced pellet coat damage have been identified and studied. These include selecting a coating polymer that can withstand the stresses exerted by compaction (Dashevsky et al., 2004), incorporating plasticizing agents in the coating materials (Dashevsky et al., 2004), determining the optimal excipients to multiparticulates ratio (Xu et al., 2016), incorporating ‘cushioning’ agents into the filler material (Vergote et al., 2002, Siow et al., 2018, Siow et al., 2019, Elsergany et al., 2020a, Elsergany et al., 2020b, Sántha et al., 2020), modifying the attributes of the multiparticulates such as porosity, size and/or composition (Lin et al., 2011, Elsergany et al., 2020b) and adjusting the compaction speed or force (Xu et al., 2016). The ability of a material to mitigate the pellet coat damage is often related to absorbing part of the compaction energy. Such materials are typically referred to as ‘cushioning’ agents, and previously investigated materials include microcrystalline cellulose (MCC), carrageenans, chitosans and alginates, among others (Schmidt et al., 2003, Picker, 2004, Schmid and Picker-Freyer, 2009, Sántha et al., 2020, Elsergany et al., 2020b). Cushioning pellets consisting of wax or crospovidone have also been studied (Vergote et al., 2002, Elsergany et al., 2020a) as they overcome issues related to flow and segregation and are inherently more pliable than the coated pellets, thereby able to absorb the compaction force in lieu of the coated pellets. Sustained release coated pellets may also be overcoated with hydroxypropyl methylcellulose (Haslam et al., 1998, Chambin et al., 2005), polyethylene glycol (Altaf et al., 1999) or MCC (Altaf et al., 1999) to enhance the mechanical integrity of the coat.
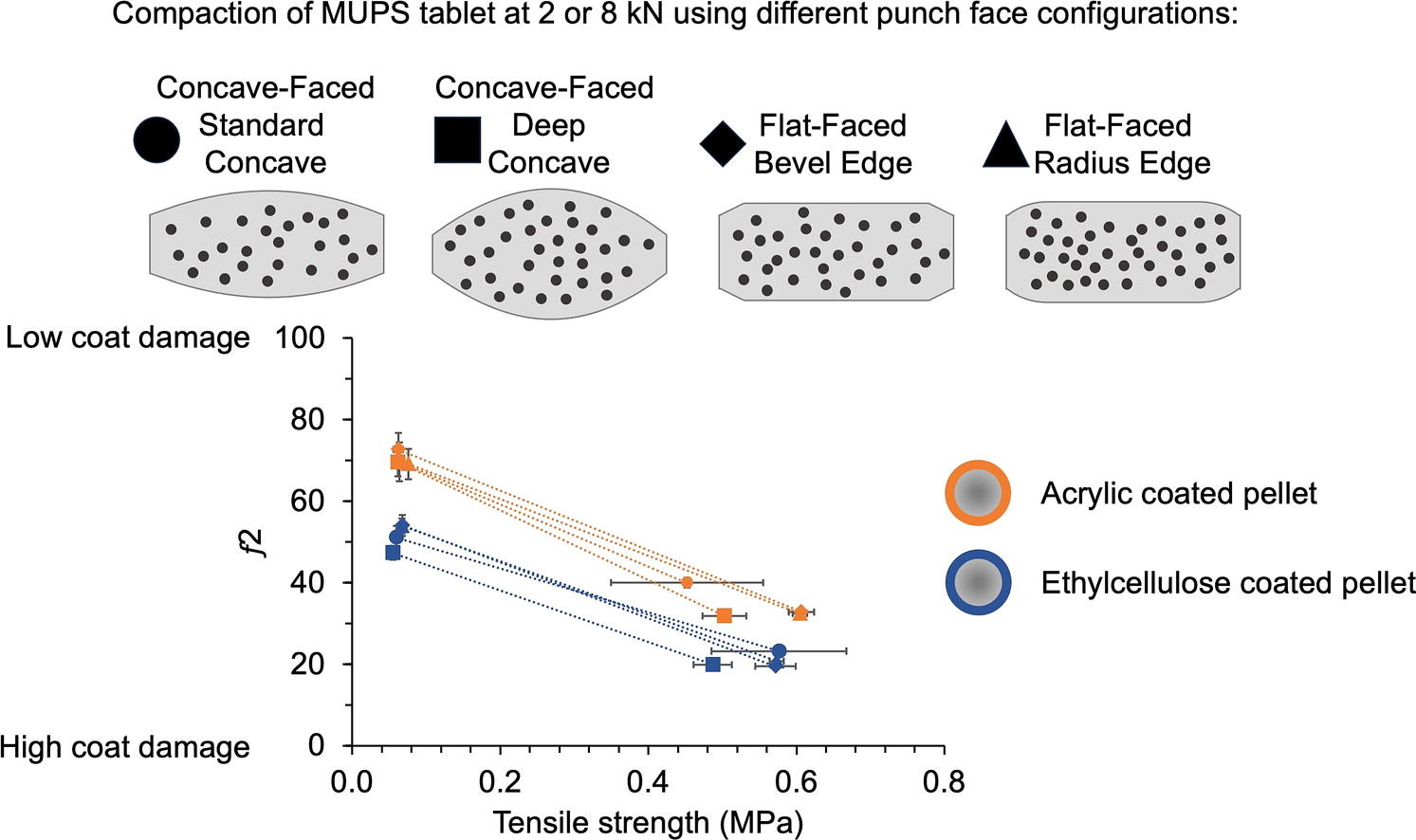
A particular interest in the production of MUPS tablets is related to the consequences of the choice of the compaction tools used. The types of punch head flats or punch faces have been reported to influence tablet properties. Tablets fabricated using flat-faced radius edge punches had higher hardness and less capping tendency than those made using flat-faced bevel edge punches (Anbalagan et al., 2017a). Using punches with a wider head flat improved the mechanical strength of tablets due to an increase in dwell time (Anbalagan et al., 2017b). Minor modifications to punch surfaces such as converting straight bevel edges to radial edges brought about greater compact densification and stronger tablets (Anbalagan et al., 2017a). Additionally, studies have shown that punches with different face configurations led to variations in powder movement and densification during compaction (Sixsmith and McCluskey, 1981, Eiliazadeh et al., 2003, Eiliazadeh et al., 2004).
The punch face configuration provides the mold to shape the tablets and is of great importance because studies have shown that tablet shape can influence patient tolerability and swallowability of the dosage form (Liu et al., 2016, Vallet et al., 2020, Shariff et al., 2020, Hummler et al., 2023). Punch faces may be fabricated with symbols or characters to imprint shapes or texts onto tablets for identification. They may also contain ridges to obtain break lines in the tablets produced and contain subtle curvatures to reduce stress distribution. The punch faces that are commonly used possess either a flat or concave shape, which may have profound influences on the physical properties of the resultant tablets.
Flat cylindrical tablets would have a more uniform distribution of the compaction force than biconvex tablets (Sixsmith and McCluskey, 1981, Diarra et al., 2015). However, the sharp peripheral edges of cylindrical tablets can be prone to breaking, potentially causing increased friability. Hence, flat-faced cylindrical tablets are always made with beveled edges. Traditionally, tablets were prepared using flat-faced bevel edge (FFBE) punches but it was later found that better quality tablets could be made using flat-faced radius edge (FFRE) punches (Anbalagan et al., 2017a). The minor change of a straight bevel edge to a curved (i.e., radius) edge appeared to improve the force distribution during compaction and as a result, better consolidated compacts were made. Biconvex tablets are also very popular, particularly when the tablets are to be coated because biconvex tablets are less prone to stacking in the coating pan during the coating process, unlike flat-faced tablets.
Although the compaction of MUPS tablets has been widely researched, there is scarce information on the impact of changing the punch face configuration. A study comparing round-flat and elliptical biconvex-shaped MUPS tablets reported slower drug release from the elliptical biconvex MUPS tablets, and it was suggested that the latter experienced less coat damage (Dreu et al., 2011). With very limited studies on how different tablet shapes or punch face configurations affect MUPS tableting, this study was initiated to investigate the influence of changing the tablet punch face configurations. As such, the following experimental design was conceived for this study. Different round punch face configurations were used to fabricate MUPS tablets, including concave-faced standard concave (CFSC), concave-faced deep concave (CFDC), FFBE and FFRE. Cross sectional diagrams of these punch face configurations are shown in Fig. 1. The tablets were produced at either low (2 kN) or high (8 kN) compaction force. Pellets coated with either ethylcellulose (EC) or acrylic (AC) were compacted with MCC as the filler excipient. The elastic recovery, ejection force, and compaction energy values were determined from the compaction simulator. After tableting, the resultant tablets were characterized for their tensile strength and the extent of pellet coat damage was assessed by comparing the drug release profiles between compacted and uncompacted pellets. An ideal punch face configuration for MUPS tablets should be capable of producing mechanically strong tablets with minimal pellet coat damage.
Read more here
Materials
The model drug was metformin hydrochloride (MET; Granules India Limited, India). Sugar cores (Suglets® 500–600 μm, Colorcon, USA) were layered with drug, followed by coating with EC or AC to produce sustained release pellets. Hydroxypropyl methylcellulose (Methocel VLV, Dow Chemical, USA) and polyvinylpyrrolidone (Plasdone C-15, Ashland, USA) were used in drug-layering onto the sugar cores. For the EC coat, aqueous EC dispersion (Surelease®, Colorcon, USA) was used.
Daniel Robin Thio, Natalia Veronica, Paul Wan Sia Heng, Lai Wah Chan, Tableting of coated multiparticulates: Influences of punch face configurations, International Journal of Pharmaceutics, 2024, 123863, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.123863.
Read more on MUPS:

