Tailoring drug release in bilayer tablets through droplet deposition modeling and injection molding
Abstract
This study explores the innovative production of personalized bilayer tablets, integrating two advanced manufacturing techniques: Droplet Deposition Modeling (DDM) and Injection Molding (IM). Unlike traditional methods limited to customizing dense bilayer medicines, our approach uses Additive Manufacturing (AM) to effectively adjust drug release profiles. Focusing on Caffeine and Paracetamol, we found successful processing for both DDM and IM using Caffeine formulation. The high viscosity of Paracetamol formulation posed challenges during DDM processing. Integrating Paracetamol formulation for the over-molding process proved effective, demonstrating IM’s versatility in handling complex formulations. Varying infill percentages in DDM tablets led to distinct porosities affecting diverse drug release profiles in DDM-fabricated tablets. In contrast, tablets with high-density structures formed through the over-molding process displayed slower and more uniform release patterns. Combining DDM and IM techniques allows for overcoming the inherent limitations of each technique independently, enabling the production of bilayer tablets with customizable drug release profiles. The study’s results offer promising insights into the future of personalized medicine, suggesting new pathways for the development of customized oral dosage forms.
Introduction
The traditional approach to medicine administration, using separate dosage forms, poses challenges in terms of inconvenience, potential medication errors, and issues with patient compliance (Khaled et al., 2015, Pereira et al., 2020, Robles-Martinez et al., 2019). In recent decades the emergence of polypharmacy propelled by the aging demographic and the surge in chronic health conditions, underscores the urgent need for innovative solutions in medication administration (Molokhia and Majeed, 2017). The concept of a customized polypill, incorporating numerous layers and compositions, offers a potential avenue for personalized drug administration (McDonagh et al., 2023b, Auriemma et al., 2022, Fuenmayor et al., 2019b, Goh et al., 2021, Goh et al., 2021, Ullah et al., 2023, Xu et al., 2023).
Despite the technological advancements and extensive research on personalized medicine, the pharmaceutical manufacturing industry continues to rely on mass-production models due to their cost-effectiveness (Andreadis et al., 2022). Mass customization aims to produce individually designed products for the patients while maintaining production cost-effectiveness (Fuenmayor, O’Donnell, et al., 2019). Three-dimensional printing (3DP) or additive manufacturing (AM) plays a pivotal role in revolutionizing the pharmaceutical manufacturing landscape, offering the ability to create customized medication forms with distinct shapes and drug release patterns not easily achievable with traditional methods (Ebrahimi et al., 2023, Ebrahimi and Ramezani Dana, 2022, Fuenmayor et al., 2018, Fuenmayor et al., 2019b, Ramezani Dana and Ebrahimi, 2023).
Droplet Deposition Modeling (DDM), a type of AM, requires feedstock material in granulate form, offering increased versatility in material options compared to conventional fused filament fabrication. This advantage can allow broader applications for DDM, making it a valuable and adaptable technology. Our earlier study assessed the drug release properties of oral dosage forms containing Hydrochlorothiazide using two AM techniques of DDM and FFF (Ebrahimi et al., 2023). The results indicated that due to the filament nature of FFF and droplet deposition nature of the DDM technique, fabricated tablets with identical infill densities had distinct microstructures, resulting in variable drug release profiles. DDM technique demonstrated satisfactory reproducibility compared to pharmacopoeia specification (McDonagh et al., 2023a). Earlier, DDM application has been explored as a novel approach to enhance the release of poorly water-soluble compounds and to achieve desired dosage levels by requiring lower drug loadings compared to conventional thermoplastic processing techniques (Welsh et al., 2019). DDM has been used for the fabrication of multiple medications using different dual-drug designs for achieving simultaneous, delayed, and pulsatile drug release regimes (McDonagh et al., 2023a, B. Zhang et al., 2021).
DDM’s layer-by-layer deposition of molten plastic allows for the customization of drug release profiles. However, challenges such as material adhesion and viscosity affect its applicability. On the other hand, injection molding (IM) is a high-production method widely used for tablet manufacturing, offering notable advantages such as time and cost savings, as well as increased efficiency (Ebrahimi et al., 2023, Fuenmayor et al., 2018, Fuenmayor et al., 2019a, Fuenmayor et al., 2019b, Walsh et al., 2022, Welsh et al., 2019, Wirth et al., 2023, Xu et al., 2023). IM is a sophisticated, solvent-free manufacturing process, allowing for the production of complex shapes with high precision and repeatability widely utilized in various industries. This method begins with the feeding of raw materials, typically in pellet or granule form, into a high-temperature chamber. Here, the material melts to form a viscous liquid and is then injected under high pressure into precisely designed molds.
Combining AM and IM techniques provides opportunities for modifying and controlling drug release profiles from a single tablet. The dosage forms manufactured through the IM method typically possess a dense structure, resulting in a slower drug release kinetic compared to tablets fabricated using AM techniques (Ebrahimi et al., 2023, Fuenmayor et al., 2019b, Qi and Craig, 2016, Tambe et al., 2021, Xu et al., 2023). Recently, researchers have investigated combining FFF and IM techniques for the fabrication of multiple drugs from a single tablet (Fuenmayor, O’Donnell, et al., 2019), and achieve the modified and controlled drug release profile through the use of bilayer solid dosage form tablets (Xu et al., 2023).
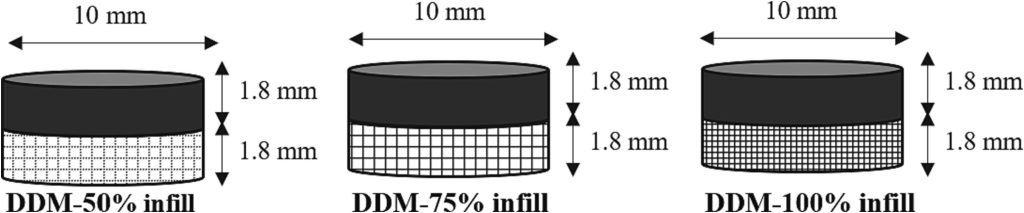
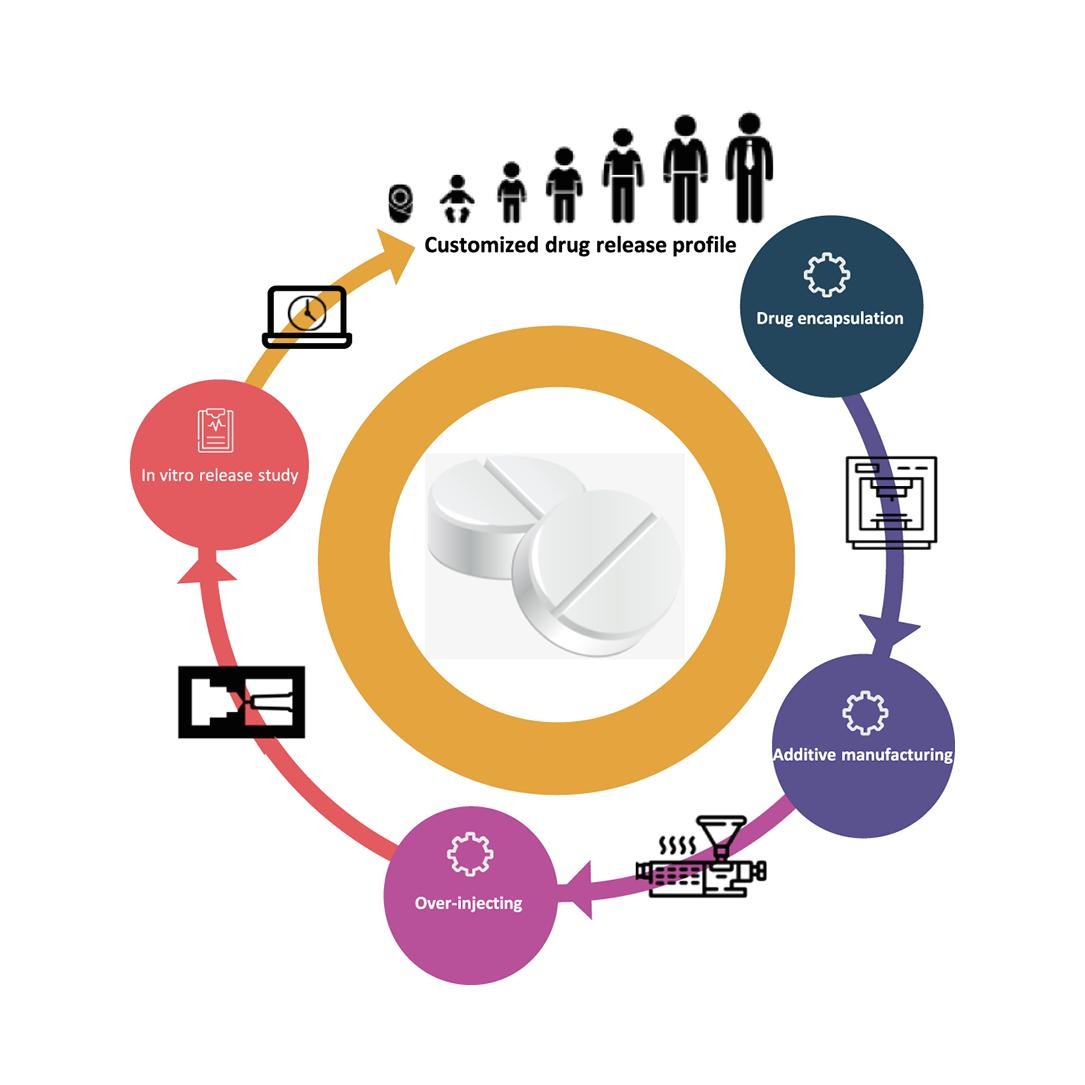
In our study, the DDM technique is employed to address the limitation in conventional tablet manufacturing pertains to the challenge of adjusting drug release properties by modifying the processing parameters, such as infill percentage. DDM’s versatility in modifying these parameters allows precise control over the drug release characteristics, a crucial aspect of personalized medicine. However, the incorporation of high-viscosity material as feedstock in DDM presents particular challenges. These challenges stem from the material properties affecting the printing process, notably the high adhesion between layers and the nozzle, and reduced adhesion to the printing platform. Addressing these challenges, our primary objective was to develop a comprehensive manufacturing platform capable of customizing polypill fabrication. This platform leverages the strengths of both DDM and IM techniques. While DDM offers the flexibility of altering infill percentages to alter the drug release properties, IM is utilized to counteract the limitations associated with DDM’s handling of incompatible feedstock viscoelastic properties. This combined approach enables the fabrication of tablets with varied structural properties, denser tablets using IM, and those with higher porosity through DDM allowing us to tailor drug release characteristics to individual patient needs. To evaluate the effectiveness of this mass customization approach, we prepared two formulations consisting of Caffeine and Paracetamol as model drugs using the HME technique. These formulations were then processed using both DDM printing and IM, adopting an over-molding methodology. Our comprehensive evaluation includes analyzing structural composition, thermal properties, melt-flow characteristics, internal structure, friability, and drug-release properties. Through these assessments, we aim to provide valuable insights into advanced pharmaceutical manufacturing techniques.
Download the full article as PDF here Tailoring drug release in bilayer tablets through droplet deposition modeling and injection molding
or read it here
Materials
Polycaprolactone (PCL) in powder form (Capa 6506, average Mw = 50,000) was purchased from Perstop (Cheshire, UK). Kollidon® VA64 (PVP/VA) was obtained from BASF Ireland (Cork, Ireland). Caffeine was purchased from VWR International (Dublin, Ireland). Both Paracetamol (acetaminophen, Batch number: MKCD6375) and Phosphoric acid were supplied from Sigma Aldrich, Wicklow, Ireland). Potassium chloride (KCL) and Hydrochloric acid (HCL) 37 % were obtained from Merck KGaA, (Darmstadt, Germany). Methanol (99.99 %, HPLC grade) was ordered from Honeywell (Seetze, Germany). Deionized water was used throughout the experiment.
Farnoosh Ebrahimi, Han Xu, Evert Fuenmayor, Ian Major, Tailoring drug release in bilayer tablets through droplet deposition modeling and injection molding, International Journal of Pharmaceutics, Volume 653, 2024, 123859, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.123859.
Read more on 3D Printing here:


