Polymeric Films Containing Tenoxicam as Prospective Transdermal Drug Delivery Systems: Design and Characterization
The administration of drugs via transdermal therapeutic systems has become an attractive form of therapeutic approach, considering its advantages and the high patient compliance achieved, making them a viable alternative, especially in the treatment of chronic diseases.
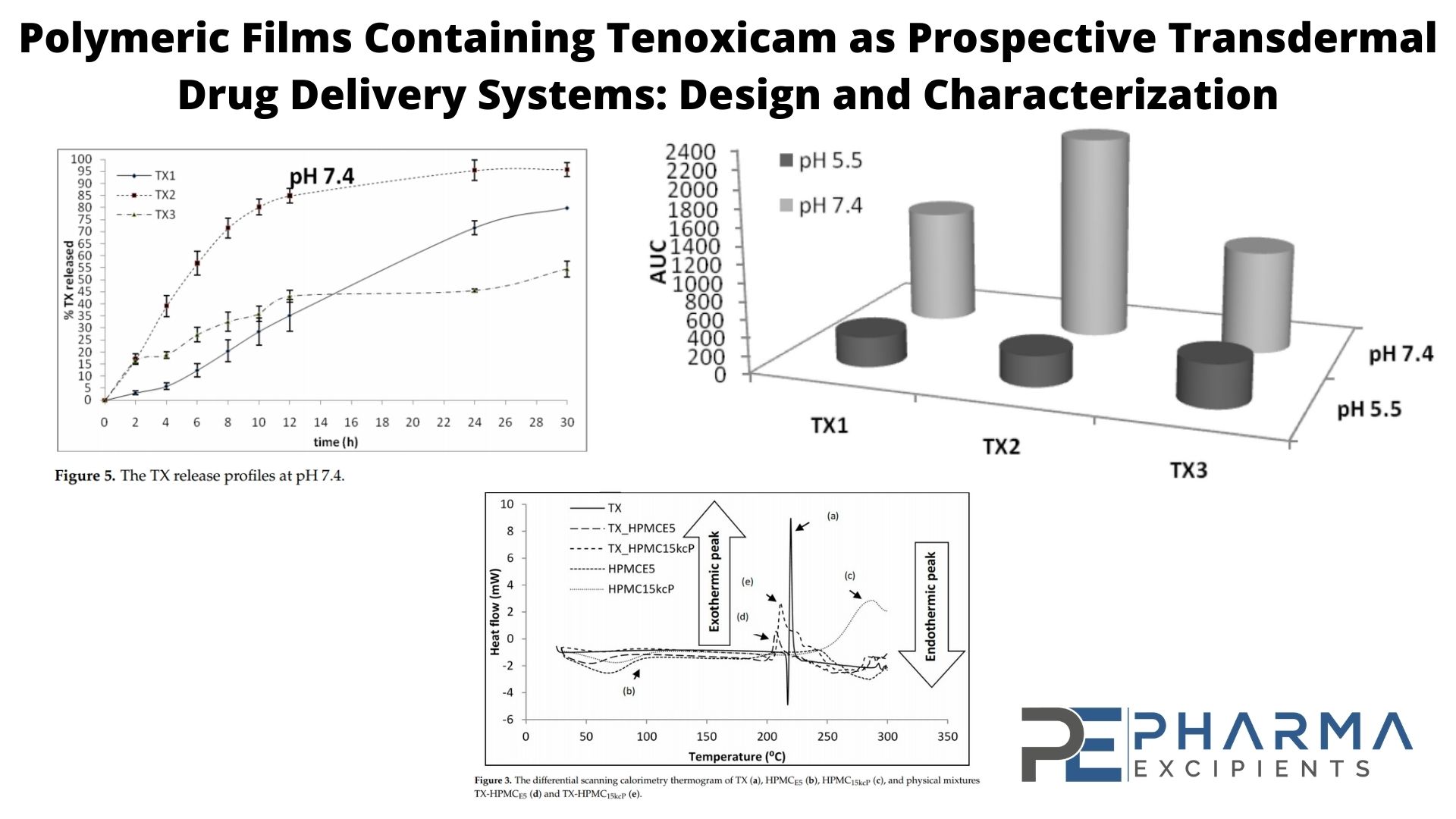
The purpose of our study was the development of polymer-based films containing tenoxicam (TX) and the analysis of dissolution kinetics. Auxiliary substances represent an important part of pharmaceutical forms, so during the first stage, TX and excipient compatibility were verified. Fourier Transform Infrared Spectroscopy (FT-IR) and Differential Scanning Calorimetry (DSC) analyses were performed on TX and on physical mixtures of TX-HPMCE5 and TX-HPMC15kcP. Three polymeric films of TX (TX1, TX2, and TX3) were prepared using a solvent evaporation technique. Release studies were done at 32 °C ± 1 °C with a Franz diffusion cell. The results of the DSC and FT-IR analyses demonstrated the compatibility of the active substance with the two matrix-forming polymers.
The results obtained in the release studies of TX from the proposed polymeric films suggested a pH-dependent behavior in all three polymeric films. At pH 5.5, flux values were between 8.058 ± 0.125 μg·cm−2·h−1 and 10.850 ± 0.380 μg·cm−2·h−1; and at pH 7.4, between 10.990 ± 0.2.490 μg·cm−2·h−1 and 53.140 ± 0.196 μg·cm−2·h−1. The Korsmeyer–Peppas model described a non-Fickian transport mechanism. The n values varied between 0.63–0.7 at pH 5.5 and 0.73–0.86 at pH 7.4, which suggested a diffusion depending on the matrix hydration and polymer relaxation.
Download the full article here: Polymeric Films Containing Tenoxicam as Prospective Transdermal Drug Delivery Systems- Design and Characterization
or continue reading here: Ciurba, A.; Antonoaea, P.; Todoran, N.; Rédai, E.; Vlad, R.A.; Tătaru, A.; Muntean, D.-L.; Bîrsan, M. Polymeric Films Containing Tenoxicam as Prospective Transdermal Drug Delivery Systems: Design and Characterization. Processes 2021, 9, 136.

