Transdermal Delivery of Pramipexole Using Microneedle Technology for the Potential Treatment of Parkinson’s Disease
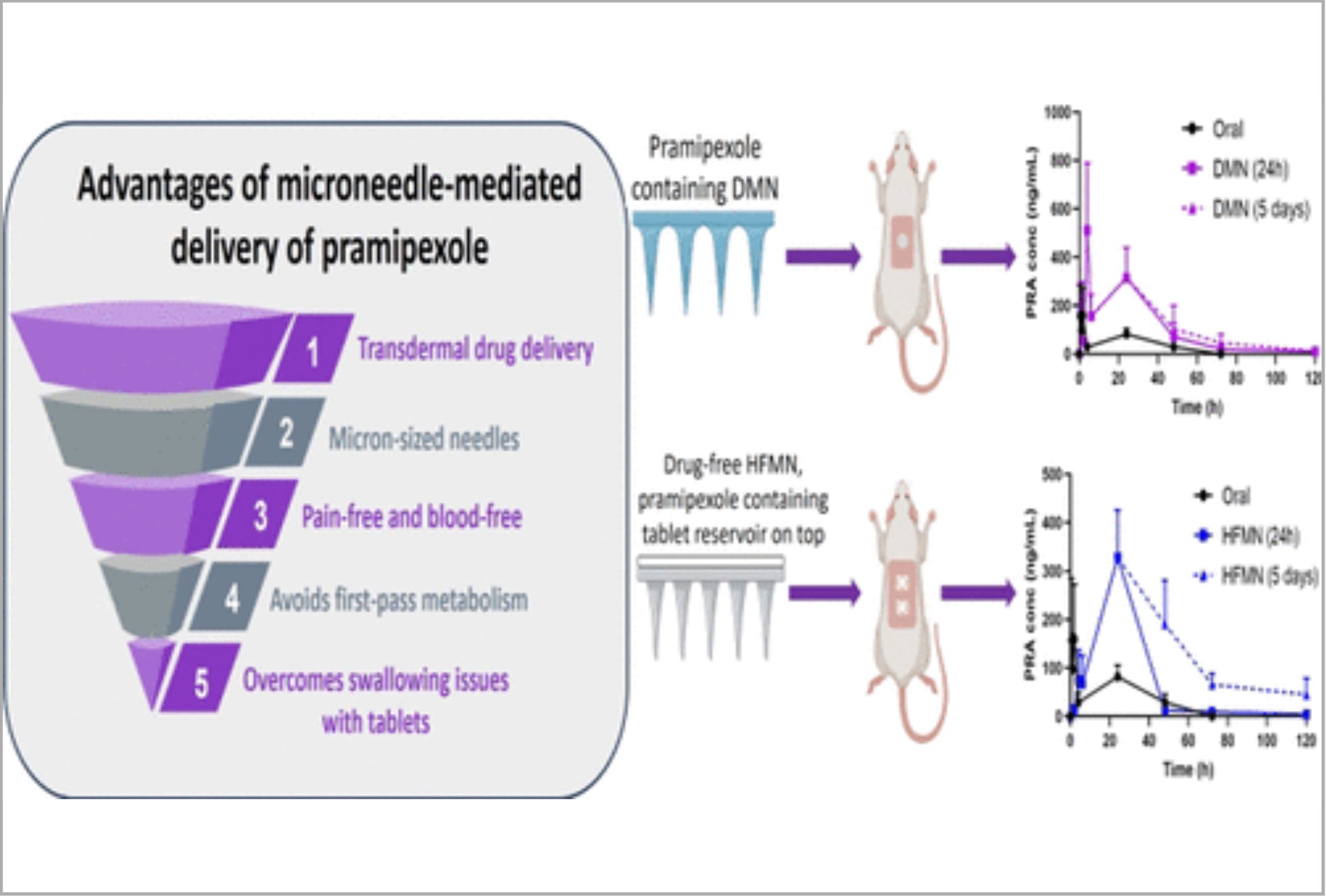
Parkinson’s disease (PD) is a debilitating neurodegenerative disease primarily impacting neurons responsible for dopamine production within the brain. Pramipexole (PRA) is a dopamine agonist that is currently available in tablet form. However, individuals with PD commonly encounter difficulties with swallowing and gastrointestinal motility, making oral formulations less preferable. Microneedle (MN) patches represent innovative transdermal drug delivery devices capable of enhancing skin permeability through the creation of microconduits on the surface of the skin. MNs effectively reduce the barrier function of skin and facilitate the permeation of drugs.
The work described here focuses on the development of polymeric MN systems designed to enhance the transdermal delivery of PRA. PRA was formulated into both dissolving MNs (DMNs) and directly compressed tablets (DCTs) to be used in conjunction with hydrogel-forming MNs (HFMNs). In vivo investigations using a Sprague–Dawley rat model examined, for the first time, if it was beneficial to prolong the application of DMNs and HFMNs beyond 24 h. Half of the patches in the MN cohorts were left in place for 24 h, whereas the other half remained in place for 5 days.
Throughout the entire 5 day study, PRA plasma levels were monitored for all cohorts. This study confirmed the successful delivery of PRA from DMNs (Cmax = 511.00 ± 277.24 ng/mL, Tmax = 4 h) and HFMNs (Cmax = 328.30 ± 98.04 ng/mL, Tmax = 24 h). Notably, both types of MNs achieved sustained PRA plasma levels over a 5 day period. In contrast, following oral administration, PRA remained detectable in plasma for only 48 h, achieving a Cmax of 159.32 ± 113.43 ng/mL at 2 h.
The HFMN that remained in place for 5 days demonstrated the most promising performance among all investigated formulations. Although in the early stages of development, the findings reported here offer a hopeful alternative to orally administered PRA. The sustained plasma profile observed here has the potential to reduce the frequency of PRA administration, potentially enhancing patient compliance and ultimately improving their quality of life. This work provides substantial evidence advocating the development of polymeric MN-mediated drug delivery systems to include sustained plasma levels of hydrophilic pharmaceuticals.
Download the full article as PDF here: Transdermal Delivery of Pramipexole Using Microneedle Technology for the Potential Treatment of Parkinson’s Disease
or read it here
Materials
PRA base (PRAB) and salt (PRAS) were purchased from Cangzhou Enke Pharma-tech Co. Ltd. (China). Acetonitrile (>99.9%), phosphate-buffered saline (PBS) tablets (pH 7.4), PVA 9–10 kDa, PVA 31–50 kDa, PEG10,000, sorbitol, and trifluoracetic acid (TFA) were purchased from Sigma-Aldrich (Dorset, UK). Gantrez S-97 and PVP (MW 58 kDa), sold under the product brand name Plasdone K-29/32, were obtained from Ashland (Worcestershire, UK). Microcrystalline cellulose (MCC) was purchased from DFE Pharma (Klever Strasse, Germany). Anhydrous citric acid and anhydrous sodium carbonate (Na2CO3) were obtained from BDH Laboratory Supplies (Dorset, UK). LCMS/MS grade methanol and formic acid were purchased from Sigma-Aldrich (Gillingham, UK), with ultrapure water (18.2MΩ-cm) produced in-house using a Millipore water purification system (Millipore, Cork, Ireland).
Mary B. McGuckin, Aaron R.J. Hutton, Ellie R. Davis, Akmal H.B. Sabri, Anastasia Ripolin, Achmad Himawan, Yara A. Naser, Rand Ghanma, Brett Greer, Helen O. McCarthy, Alejandro J. Paredes, Eneko Larrañeta, and Ryan F. Donnelly, Transdermal Delivery of Pramipexole Using Microneedle Technology for the Potential Treatment of Parkinson’s Disease, Cite this: Mol. Pharmaceutics 2024, Publication Date:April 11, 2024, https://doi.org/10.1021/acs.molpharmaceut.4c00065, © 2024 The Authors. Published by American Chemical Society. This publication is licensed under CC-BY 4.0.
Read also our introduction article on Microcrystalline Cellulose here:


