Strategies to Enhance the Solubility and Bioavailability of Tocotrienols Using Self-Emulsifying Drug Delivery System
Abstract
Tocotrienols have higher medicinal value, with multiple sources of evidence showing their biological properties as antioxidant, anti-inflammatory, and osteoprotective compounds. However, tocotrienol bioavailability presents an ongoing challenge in its translation into viable products. This is because tocotrienol oil is known to be a poorly water-soluble compound, making it difficult to be absorbed into the body and resulting in less effectiveness. With the potential and benefits of tocotrienol, new strategies to increase the bioavailability and efficacy of poorly absorbed tocotrienol are required when administered orally. One of the proposed formulation techniques was self-emulsification, which has proven its capacity to improve oral drug delivery of poorly water-soluble drugs by advancing the solubility and bioavailability of these active compounds. This review discusses the updated evidence on the bioavailability of tocotrienols formulated with self-emulsifying drug delivery systems (SEDDSs) from in vivo and human studies. In short, SEDDSs formulation enhances the solubility and passive permeability of tocotrienol, thus improving its oral bioavailability and biological actions. This increases its medicinal and commercial value. Furthermore, the self-emulsifying formulation presents a useful dosage form that is absorbed in vivo independent of dietary fats with consistent and enhanced levels of tocotrienol isomers. Therefore, a lipid-based formulation technique can provide an additional detailed understanding of the oral bioavailability of tocotrienols.
Introduction
Vitamin E is a fat-soluble antioxidant that can only be produced by plants through photosynthesis, hence it needs to be obtained from foods or supplements, as it plays a crucial role in human nutrition. The distribution of vitamin E in palm oil was reported to be approximately 30% tocopherols and 70% tocotrienols [1]. Tocotrienols have similar structures to tocopherols, with a chromanol head denoted by α, β, γ, or δ, which depends on the position number and methyl groups on the chromanol ring [2]. Tocotrienols differ structurally from tocopherols by the degree of saturation at the side chains, with three double bonds at carbons 3, 7, and 11, whereas tocopherols have saturated phytyl side chains. It was discovered to naturally occur at higher levels in certain cereals and vegetable oils, including palm oil, rice bran oil, barley germ, wheat germ, and annatto [3,4,5,6,7].
Tocotrienols are valuable nutraceuticals due to their numerous pharmacological properties, particularly in preventing or treating non-communicable diseases, including cardiovascular, musculoskeletal, metabolic, and skin disorders, as well as cancers [8]. Tocotrienols have biochemical functions that are directly or indirectly impacted by their antioxidant properties, which prevent the non-enzymatic oxidation of various cell components by molecular oxygen and free radicals. In several studies, tocotrienols have been shown to inhibit free radical production via the induction of enzymes such as superoxide dismutase [9,10] and glutathione peroxidase, which neutralise superoxide radical-produced free radicals [11]. In addition, tocotrienols have been extensively studied for their anti-inflammatory properties, and promising scientific evidence has been presented. In light of the fact that inflammation is a full array of physiological responses to a foreign organism, it has been established that inflammation is a significant element in the progression of numerous chronic diseases and disorders. Tocotrienols have been demonstrated to inhibit the expression of inflammatory mediators such as tumour necrosis factor-alpha [10], interleukin [IL]-1 [12], IL-6 [13], and nitric oxide synthase [14]. Antioxidants protect tissues from damage against reactive oxygen species and other free radicals, indirectly preventing unwanted inflammatory responses from occurring in the first place.
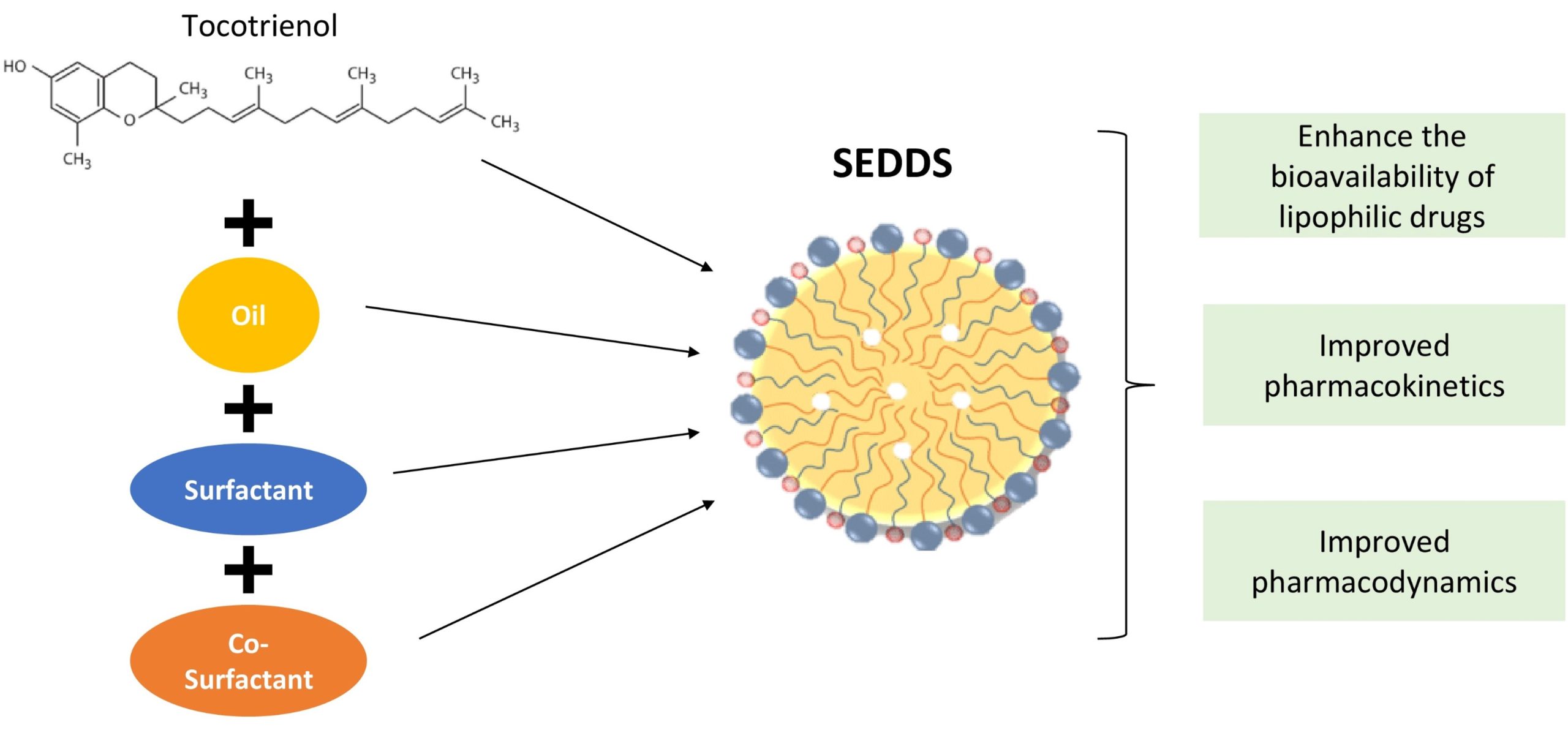
Despite their medicinal value, translating tocotrienols into a viable medicinal product remains challenging due to their low oral bioavailability. Oral administration remains the preferred choice for drug delivery due to its safety, patient compliance, and self-administration capacity. Although oral delivery is the most convenient, it is also limited by a number of barriers in the gastrointestinal tract (GIT) [15]. Upon oral administration, drug solubilisation in the GIT is essential for absorption, as insufficient dissolution can cause incomplete absorption, low bioavailability, and high variability [16]. Tocotrienol has unfavourable physicochemical properties as a highly viscous oil that is nearly insoluble in water and readily oxidised by atmospheric oxygen. Other limitations exist, such as saturable intestinal absorption and the selectivity of the transfer protein [17,18]. These physicochemical, biological, physiological, and anatomical factors act independently and in concern to limit tocotrienol bioavailability and prevent effective oral delivery [19]. Using a rat model, an in vivo study found that intraperitoneal and intramuscular administration of tocotrienols resulted in minimal absorption, whereas oral administration resulted in incomplete absorption of tocotrienols [20]. Researchers have invented various methods to improve the bioavailability of these compounds. One of the innovations is a self-emulsifying drug delivery system (SEDDS), an isotropic mixture of oils, surfactants, co-surfactants, and co-solvents. SEDDSs can improve the oral absorption of highly lipophilic drug compounds by maintaining them in a solubilised state, thereby preventing an improperly water-soluble compound from solubilising and subsequently dissolving [21].
The oral bioavailability of each tocotrienol form was reported low in previous studies, with α-tocotrienol at 27.7%, γ-tocotrienol at 9.1%, and δ-tocotrienol at 8.5% [22]. Therefore, without α-tocopherol, tocotrienol absorption is virtually nonexistent without suitable conditions and optimal fat levels. The findings also showed that the elimination half-lives of several tocotrienol forms ranged between 2.3 and 4.4 h, much shorter than α-tocopherol elimination half-lives, which lasted 48 to 72 h [23,24,25]. The poor and inconsistent oral bioavailability of fat-soluble compounds in the GI led researchers to explore solutions to overcome these issues and ensure positive therapeutic effects in humans.
In this review, the composition and formulation of tocotrienols using SEDDSs, as well as study populations on tocotrienol bioavailability, will be discussed. Tocotrienols have been the subject of a number of human studies over the past decade. However, many factors complicate tocotrienol bioavailability and remain unanswered.
Download the full article as PDF here Strategies to Enhance the Solubility and Bioavailability of Tocotrienols Using Self-Emulsifying Drug Delivery System
or read it here
Excipients mentioned in the study: Vitamin E TPGS, TPGS, chitosan, PLGA
Mohamad, N.-V. Strategies to Enhance the Solubility and Bioavailability of Tocotrienols Using Self-Emulsifying Drug Delivery System. Pharmaceuticals 2023, 16, 1403. https://doi.org/10.3390/ph16101403
See the webinar:
“Enhanced Stability and Bioavailability of Poorly Soluble APIs“,
18 October 2023:
Get more information & register here for free:


