Unveiling the potential of microsponges: Enhancing oral bioavailability
Abstract
Introduction
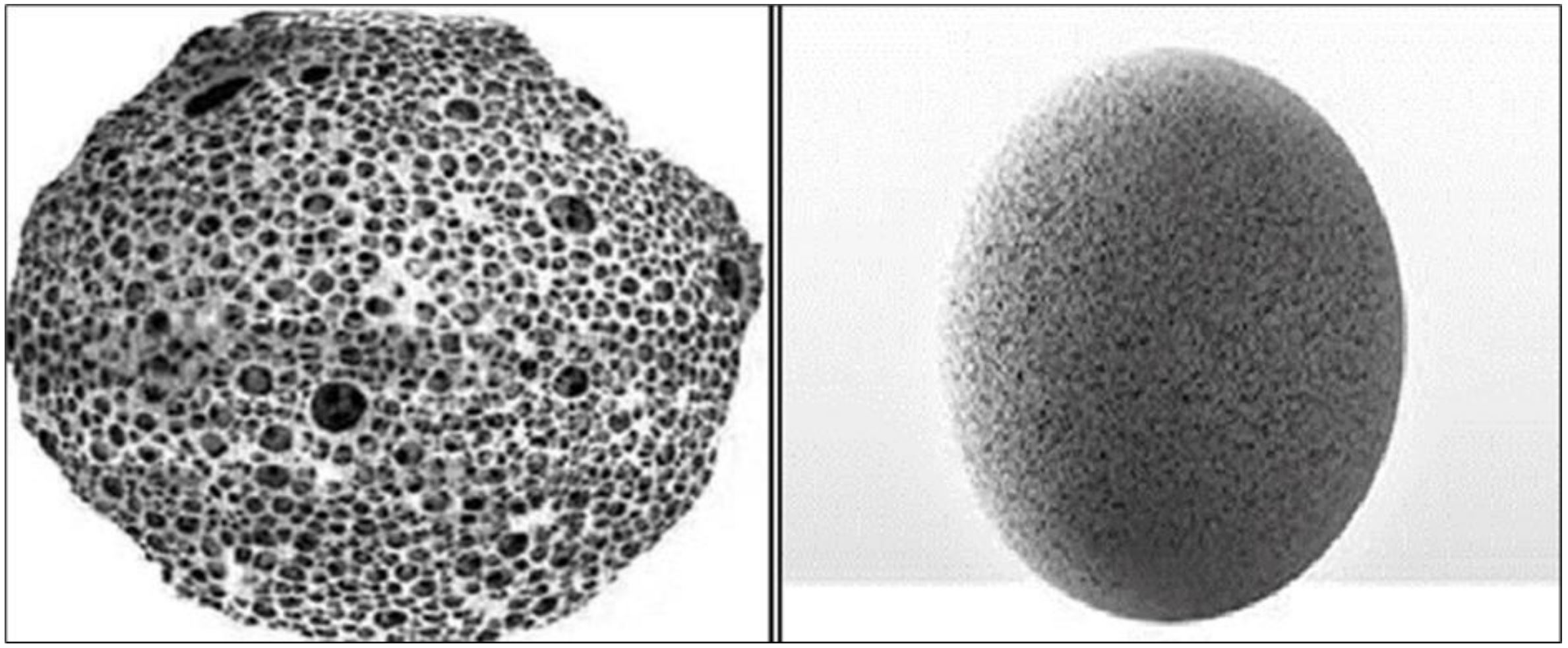
Microsponges are porous, non-collapsible, highly cross-linked polymeric microspheres microsponges with a particle
size range of 5 to 300 μm. They can absorb a broad variety of active substances and release them gradually.
Microsponges’ sponge-like texture gives them special dissolving and compression characteristics. They have little
negative effects and increase patient compliance. They are also very stable, non-toxic, non-allergic, non-mutagenic, and extremely effective. Microsponges made of a variety of polymers, including PHEMA, ethyl cellulose, polystyrene, and Eudragit RS100, have been used. Moreover, these active microsponges offer a wide range of advantages and can be added to formulations including capsules, gel, and powders. Microsponges have shown promise in the fields of
cosmetics and pharmaceuticals. Examples of their applications include antifungal vaginal gel, enhanced arthritis
therapy, burn wound treatment using silver sulfadiazine-loaded microsponge gel, gastro retentive delivery, matrix
tablets, and colon-specific drug delivery systems.
Patented polymeric delivery systems called microsponges are made of porous microspheres that can hold a variety of
active substances, including sunscreens, emollients, perfumes, essential oils, and anti-infective, anti-fungal, and antiinflammatory compounds. Each microsphere has a huge porous surface area and is made up of numerous
interconnected voids within a non-collapsible structure, much like a real sponge. Won invented the microsponge
technique in 1987, and Advanced Polymer Systems was given the original patents.
Currently, Cardinal Health, Inc. holds a license to utilize this intriguing technology for topical products. Depending on
the level of smoothness or after-feel needed for the final recipe, the microsponges’ diameter can range from 5 to 300
μm. A typical 25 μm sphere can have up to 250000 holes and an internal pore structure similar to 10 feet in length,
meaning that even though the microsponge size may vary, the total pore volume will be approximately 1 ml/g. As a
result, each microsponge develops a sizable reservoir that can hold up to its own weight’s worth of active agent. These
microsponge materials are made safer by the fact that the microsponge particles themselves are too big to penetrate
the skin. The possibility of bacterial contamination of the materials trapped in the microsponge is another issue related
to safety. Because the pore width is smaller, bacteria with a size range of 0.007 to 0.2 μm cannot enter the microsponges’ tunnel structure.
Download the full article as PDF here: Unveiling the potential of microsponges
or read it here
Vidya K P, E. Gopinath, Ganesh N. S, J Adlin Jino Nesalin and Vineeth Chandy, Unveiling the potential of microsponges: Enhancing oral bioavailability, World Journal of Biology Pharmacy and Health Sciences, 2024, 17(02), 405–414. Article DOI: 10.30574/wjbphs.2024.17.2.0085, DOI url: https://doi.org/10.30574/wjbphs.2024.17.2.0085, Received on 12 January 2024; revised on 20 February 2024; accepted on 23 February 2024
CPHI & PMEC China will be held at 19-21 June 2024. Register for free:



