Implementation of Pharmaceutical Quality by Design in Wet Granulation
Function and Popularity of Wet Granulation
Granulation is a typical unit operation of particle enlargement carried out by the pharmaceutical industry to produce pharmaceutical dosage forms like tablets and capsules for over 70 years (Parikh, 2009). The purpose of wet granulation can be, but is not limited to:
- • Enhance the uniformity of drug substance in the final dosage form
- • Increase the density of blend
- • Increase the flowability and compressibility of the powder mixture
- • Reduce the dust during manufacturing process
- • Alter the physical appearance or surface properties
- • Improve the wettability of a poorly soluble drug substance
Generally, the wet granulation process commences after fully mixing the drug substance and other necessary intra-granular excipients to ensure the uniform distribution of each ingredient. The granule size used in pharmaceutical industry is usually controlled between 0.2 mm and 4 mm, with narrow particle size distribution. Drying and milling are required before tablet compression or capsule filling (Parikh, 2009; Shanmugam, 2015).
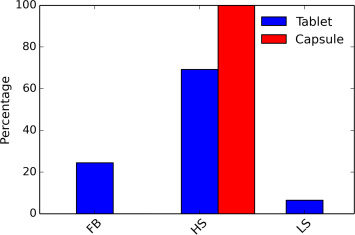
The pharmaceutical industry prefers direct compression/encapsulation over granulation. Wet granulation is to be considered next if the physical properties of final powder mixture cannot satisfy the requirement for direct compression or encapsulation. In order to understand how often pharmaceutical companies employ wet granulation in their manufacturing process to produce drug products in solid oral dosage forms, we conducted a survey by counting any type of wet granulation employed in the manufacturing processes from 98 approved new drug product applications (NDAs) and 128 approved abbreviated new drug product applications (ANDAs). Among these 226 applications, 73.9% of drug products are in tablet dosage form and the remaining 26.1% are in capsule dosage form. Among these, 46.7% of the tablets and 18.6% of the capsules employed at least one wet granulation step in the manufacturing process.
More on the implementation of pharmaceutical quality by design in wet granulation
Have a look at our other wet granulation articles:
A Quality By Design Approach to Scale-Up of High Shear Wet Granulation Process
Article Information: Xiang Yu, Lawrence X. Yu, Yue Teng, Dhaval K. Gaglani, Bhagwant D. Rege, Susan Rosencrance

