Delivery of Poorly Soluble Drugs via Mesoporous Silica: Impact of Drug Overloading on Release and Thermal Profiles
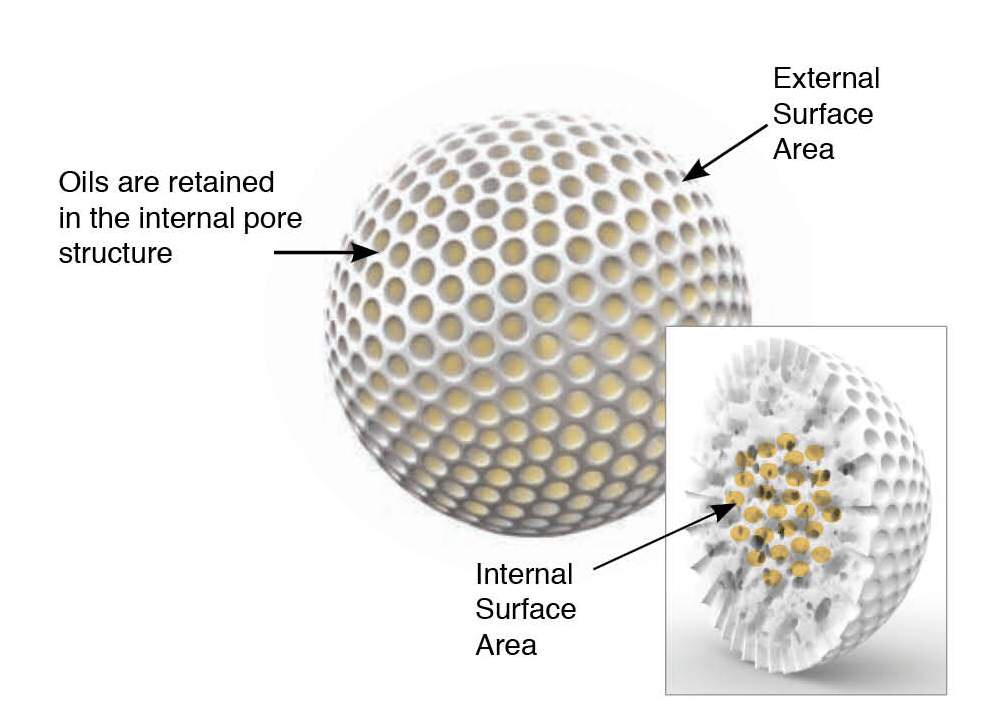
The most important properties of promising drug candidates for oral dosage form are aqueous solubility and intestinal permeability. Over 40% of drugs on the market are BCS class II and IV, which have low solubility. Furthermore, new chemical entities are even less soluble compared to marketed products with a projection of up to 70–90% of drug candidates in the pipeline suffer from low solubility [1]. Following oral administration, drugs must dissolve in gastrointestinal fluids in order to be absorbed into the systemic circulation and exert a therapeutic action. The formulation development of low solubility drugs (BCS class II and IV) faces a great challenge as these drugs are poorly absorbed and usually exhibit subsequent low or variable oral bioavailability [2].
The problem of low solubility can be addressed by using solubilisation techniques, namely solid dispersion systems, size reduction, salt formation, prodrug, liposomes, etc. Among those techniques, solid dispersions are preferred by the industry due to their practicality and low cost. This technique is mainly based on a so-called “amorphisation”, whereby the crystalline drugs are converted into their high energy amorphous form, which exhibits a superior solubility in comparison with that of the original ones [3].
Mesoporous materials, e.g., mesoporous silica, a subclass of solid dispersion systems, are considered highly effective for drug amorphisation due to their ability to achieve the spatial confinement of drug molecules within their nanometre-scale pore structure [4]. Shen et al. [5] suggested that drugs would exist in an amorphous state within the mesoporous silica if the pore size were smaller than 12 times the drug’s molecular size. Mesoporous carriers also offer formulation flexibility due to tunable pore size and surface area [6]. Furthermore, this approach has shown applicability for both existing poorly soluble drugs and drug candidates in the pipeline with various chemical structures. Utilising mesoporous silica to enhance the bioavailability of poorly soluble drugs has been successfully demonstrated in various clinical studies conducted on rabbits, dogs, and mice [7].
For loading a drug into mesoporous silica, there are several techniques, which can be categorised into two main approaches: solvent-free methods and solvent-based methods. Solvent-free methods are comprised of physical mixing followed by heating to melt the drug, co-milling between the drug and mesoporous materials, and using supercritical carbon dioxide. Although solvent-free loading methods offer apparent advantages, e.g., no requirement for checking the residual solvent in drug products and low environmental impact, these methods are still under investigation to exhibit better performance in terms of the loading efficiency and stability of thermolabile drugs. On the other hand, solvent-based approaches offer a practical and straightforward solution for drug amorphisation within mesoporous silica. Simply put, a drug is dissolved in a suitable solvent, e.g., ethanol, then mixed/impregnated with mesoporous silica. The solvent can be removed by appropriate drying techniques at the end of the process. There are various factors that influence drug loading into mesoporous silica, such as the type of solvent, drug load, accessible surface area, and the pore volume of the mesoporous silica. In general, solvent-based loading techniques, especially spray drying, produce drug-loaded mesoporous silica with a high loading efficiency compared to solvent-free techniques [6].
However, higher drug loadings above 30% (w/w) could lead to incomplete amorphisation, i.e., a small amount of crystalline drug will remain on the exterior surface of the generated particles [5]. Drug molecules can theoretically adsorb onto the silica surface of mesopores as a monolayer or multilayers, depending on the drug’s molecular dimension, accessible surface area, and pore size [8]. Dening and Taylor [9] studied ritonavir–loaded–mesoporous silica at various drug loads of a 25–150% monolayer surface coverage and found that drug release decreased significantly as the drug load increased. Therefore, it would be prudent to systematically investigate the impact of drug loading beyond monolayer surface coverage (overloading) on the thermal behaviour of drug within mesoporous silica. Furthermore, the addition of a precipitation inhibitor to the mesoporous silica has been previously investigated to overcome this recrystallisation challenge. Lainé et al. [10] found that hypromellose acetate succinate (HPMCAS), when combined with mesoporous silica, promoted a complete amorphisation of Celecoxib. However, the advantage of such ternary system combining mesoporous silica with drug and precipitation inhibitors still requires further study, to confirm whether poorly soluble drugs with different chemical natures and crystallisation tendencies can benefit from it.
Felodipine and Furosemide are BCS class II and class IV drugs, respectively. Felodipine is mainly absorbed in the small intestine [11], i.e., and alkaline pH environment, while the stomach is the favoured absorption site for Furosemide [12], i.e., acidic pH environment. Hence, Felodipine and Furosemide were selected as model drugs in this study to represent two scenarios after oral administration in drug release from mesoporous silica.
The aim of this study was to investigate the impact of drug overloading within mesoporous silica at 100–300% of the theoretical monolayer’s surface coverage on the release behaviour and thermal properties of loaded drugs. This included investigating the release profiles of the model drugs after loading within mesoporous silica alone and in combination with HPMCAS. Thermal profiles and particle morphology were also studied to confirm the amorphous/crystalline nature of the drug within the carrier system and the presence or lack of surface crystals. These studies will help elucidate the link between theoretical drug load and important formulation properties, such as loading efficiency, release behaviour, and the nature of the drug within mesoporous carriers. Download the full MDPI article here: delivery-of-poorly-soluble-drugs-via-mesoporous-silica-impact-of-drug-overloading-on-release-and-thermal-profiles.pdf
More on Grace mesoporous Silicas

